- DOI: 10.31509/2658-607x-202471-142
COMPARATIVE ASSESSMENT OF THE DECOMPOSITION RATE OF PLANT LITTERFALL IN SPRUCE AND PINE FORESTS AT THE NORTHERN DISTRIBUTION LIMIT
Original Russian Text © 2023 E. A. Ivanova, M. A. Danilova, V. E. Smirnov, V. V. Ershov published in Forest Science Issues Vol. 6, No. 3, Article 132.
E. A. Ivanova1, 2, M. A. Danilova2, V. E. Smirnov2, V. V. Ershov1
1Institute of North Industrial Ecology Problems KSC RAS
Akademgorodok st.14a, Apatity, Murmansk region, 184209, Russia
2Centre for Forest Ecology and Productivity of the Russian Academy of Sciences,
Profsoyuznaya st. 84/32 bldg. 14, Moscow, 117997, Russia
*E-mail: ea.ivanova@ksc.ru
Received: 16 August 2023
Revised: 19 September 2023
Accepted: 20 September 2023
A comparative assessment of the processes of the initial stages of decomposition of plant residues (pine needles, spruce needles, leaves of boreal shrubs, moss thalli) in lichen-shrub pine forests and shrub-green-moss spruce forests formed under natural conditions at the northern limit of distribution was carried out. The characteristics of the litterfall initial composition, the rate of decomposition, and changes in the chemical composition of plant residues in the process of destruction caused by the forest type were studied. The higher initial content of Corg in the plant tissues of pine forests is associated with favourable lighting conditions under the forest canopy, while the high content of Mn in the tissues of ground cover plants in spruce forests is due to the direct influence of spruce needle litterfall rich in this nutrient. The results of the study clearly demonstrated that the forest type has a significant impact both on the initial quality of the litterfall of the same plant species and on the rate of decomposition: in the spruce forest, spruce needles and lingonberry leaves with a higher content of nutrients (Mg, Mn, P) and narrow ratios of elements (C:N, C:P) were characterised by more active decomposition processes. However, green moss litter, despite its high quality in spruce forests, decomposed more actively in pine forests, which may be due to a large amount of precipitation in pine forests. Thus, differences in the rate of decomposition of plant residues are influenced by a combination of the plant material quality, temperature conditions, and precipitation amount associated with the forest type.
Keywords: forest type, decomposition of litter, plant residues, quality of litter
The litterfall of woody plants and ground cover plants, such as leaves, needles, buds, shoots, fruits, roots, etc., dying off over a certain period of time, is one of the most important components affecting the formation of biogeochemical cycles in forest ecosystems. Plant litterfall is a source of organic carbon and mineral nutrition elements that become available to biota during its decomposition and mineralization.
The decomposition rate of litterfall and carbon entry into the soil largely depend on the hydrothermal conditions of soils (Kuznetsov, 2010; Kuznetsov, Osipov, 2011), the activity and composition of soil biota (Vorobyova, Naumova, 2009; Högberg et al., 2017), the fractional composition of incoming litterfall (Bobkova, 2000; Fang et al., 2015), and climatic conditions (air temperature and precipitation) (Pausas, 1997; Portillo-Estrada et al., 2016). According to modern concepts, climate is considered the leading factor on a regional scale, while the quality of litterfall is considered local, whereas the activity of destructive organisms is regulated by climate and the quality of litterfall (Bradford et al., 2016) determined by concentrations of nutrients and secondary metabolites. Thus, at the early stages of decomposition, N, P and water-soluble organic compounds have the greatest effects, whereas the main determinant of decomposition dynamics at later stages is lignin (Berg, 2000; Wardle et al., 2003; Zhang et al., 2008; Rahman et al., 2013; Larionova et al., 2017). The stoichiometric ratios of elements and substances (C:N, lignin:N) in decomposing litterfall are also related to the quality of plant material: the narrower is the ratio, the higher is the rate of destruction (Berg, McClaugherty, 2008; Rahman et al., 2013; Tu et al., 2014; Lukina et al., 2017). The initial chemical composition of the litter, which determines its quality, and, accordingly, the process of its decomposition, exhibits specific features. In four different species of pine (Pinus pinea, P. laricio, P. sylvestris and P. nigra), the decomposition rate was regulated by the initial content of nutrients (N, K, Mn) (De Marco et al., 2007). Pine needles (P. contorta) contained more C and N and less lignin compared to spruce needles (Picea engelmannii), which indicates a higher quality of plant material, but carbon losses were higher for spruce (Leonard et al., 2020). Another study showed that spruce needles litterfall with a higher content of nutrients and relatively narrow C:N and lignin:N ratios decomposed noticeably faster than the pine needles litter, whereas the litterfall of silver birch leaves (Betula pendula) in pine forests, with a lower ratio of N:P, decomposed faster compared to the downy birch litterfall (B. pubescens) in spruce forests (Ivanova et al., 2019).
Woody plants are able to form forest types with their own special habitat conditions and soil fertility, namely through litter the chemical composition of which affects microbial activity and soil composition (Rakhleeva et al., 2011; Aponte et al., 2013; Chavez-Vergara et al., 2014; Kolmogorova, Ufimtsev, 2018; Pomogaybin, Pomogaybin, 2018; Tsandekova, 2018). The canopy of the forest stand, depending on the species composition and structure and closeness of the crowns, changes the composition of atmospheric precipitation and affects the level of illumination (Lukina et al., 2008; Kishchenko, 2019). Forest ecosystems in the Murmansk region represent the stages of succession, during which there is a change of tree species (pine, spruce, birch), which forms different environmental conditions in ecosystems on soil-forming rocks of similar composition. Thus, spruce, to a greater extent than pine, acidifies sediments passing through the crowns and prevents them from penetrating under the crowns of trees, creating a more pronounced mosaic. Due to the greater closeness of the crowns, spruce prevents sunlight from penetrating under the forest canopy. In spruce forests, soil fertility is significantly higher than in pine forests, which are formed on the same soil-forming rocks and positions in the landform (Lukina et al., 2002, 2006, 2008, 2010; Tsvetkov, 2004; Orlova et al., 2011). This can also determine differences in the composition of the litterfall of the same plant species in different types of forests and affect the rate of their decomposition.
The purpose of this paper is to assess the influence of forest type on the initial composition and decomposition rate of litterfall in the spruce and pine forests dominant in the North Taiga subzone.
MATERIALS AND METHODS
The research was carried out at permanent observation sites (POS) in the Murmansk region in lichen-shrub pine forests (10P) on illuvial-ferruginous podzols (Rustic podzols) in 1997–1999 and in green-mossy-shrub spruce forests (8S2B) on illuvial-humus podzols (Carbic podzols) under automorphic conditions in 1996–1998. More detailed descriptions of the objects of research are given in early papers (Lukina, Nikonov, 1996, 1998; Lukina et al., 2017; Ivanova et al., 2019). The main soil-forming rocks in the study area are glacial deposits—moraine and water-glacial—as well as sandy and sandy loam rocks according to granulometric composition (Belov, Baranovskaya, 1969; Pereverzev, 2004). During the succession of coniferous forests, when pine forests are replaced by spruce forests, patterns of changes in the chemical composition of soils are observed: in spruce forests, the content of available compounds of biophilic elements in the entire soil profile increases, while the C:N ratio decreases in organogenic horizons and humus accumulation is observed. In pine forests, the organogenic horizons of the undercrown spaces are characterised by higher acidity than those of the intercrown ones, whereas in spruce forests, the opposite pattern is observed. An increase in the content of acidic components is observed in the mineral horizons of soils, in particular, an increase in the content of fulvic acids was noted in the illuvial horizons with a decrease in the content of humic acids (Orlova et al., 2011).
Experiments to study the processes of decomposition of litterfall on POSs in different types of forests were carried out in compliance with the uniformity of methods and statistical data processing. Samples of the active fraction of the litterfall (leaves and needles) of the dominant vascular plant species (Pinus sylvestris, Picea abies, Vaccinium vitis-idaea, V. myrtillus and Empetrum hermaphroditum) and samples of the ageing brown part of mosses (Pleurozium schreberi) were taken in September 1997 at a monitoring station in pine forests, and in October 1996 in spruce forests. For decomposition, plant material (10 g of dry matter) was placed in bags made of synthetic material with a pore size of 30 μm, which were placed on the soil surface (in the litter L subhorizon) in the undercrown and intercrown spaces at monitoring sites. Samples were taken annually in October, 1 and 2 years after the start of each experiment. In just two selection periods, 32 packages were selected in pine forests, and 49 packages were selected in spruce forests.
Before chemical analysis, the plant material was milled and subjected to wet oxidation with concentrated HNO3. The concentrations of metals (Ca, Mg, K, Mn) were determined by atomic absorption spectrometry on the Aanalyst 800. For content determination, the following methods were used: the Kjeldahl method for the total nitrogen content, the Tyurin method for the organic carbon (Corg), and colorimetry for the phosphorus (Vorobyova, 1998). The lignin content was determined by treatment a sample of H2SO4 (72%) after pre-boiling in a solution of cetyltrimethylammonium bromide in a 0.5 M solution of H2SO4 (Rowland, Roberts, 1994).
Calculations were done for the absolute dry weight. The mass loss was calculated as the difference between the weight of the samples before laying and 1 or 2 years after and expressed as a percentage. The enrichment coefficient demonstrating the change in the composition of plant material during decomposition was calculated for each element as the ratio of concentration after the first or second year of decomposition to the initial concentration. Losses of nutrition elements and lignin, taking into account the rate of mass loss, were expressed as a percentage and calculated as the difference between the products of the concentration of the component per weight of the sample before the experiment and 1 or 2 years after, respectively. The quality of the litterfall was characterised based on the content of lignin (secondary metabolites), nutrients (N, Ca, Mg, K, P, Mn), and stoichiometric ratios of C:N, C:P, lignin:N, and N:P.
The statistical analysis of the data was carried out using various methods. The influence of forest type (spruce and pine) on the initial chemical composition of plant material was analysed for individual plant species using the V-test (Husson et al., 2017) in the statistical programming environment R (R Core Team …, 2017). At the same time, the data matrix for the chemical composition of the litterfall was supplemented with available data on the composition of living plants: pine and spruce needles in their last years of life, perennial leaves of lingonberry, crowberry, and perennial and dead shoots of green mosses P. schreberi and Hylocomium splendens. The decomposition parameters (loss of mass and elements, enrichment coefficient) in pine and spruce forests were compared for each fraction of the litterfall (pine and spruce needles, lingonberry and crowberry leaves, mosses) using the Mann-Whitney U-test in the Statistica program. The possible influence of intra- and interbiogeocenotic variability of soil and air temperatures in spruce and pine forests was analysed using data obtained in the period from 2015 to 2021 using temperature loggers under tree crowns, in intercrown spaces, and on trees.
RESULTS AND DISCUSSION
The initial composition of plant material—non-decomposed litterfall and living plants (their perennial parts)—characterises the quality of plant material for subsequent decomposition by its destructor organisms.
A comparison of the composition of individual fractions of the litter, as well as living plant material of the corresponding plant species, showed that spruce needles in the last years of life are richer in nutrients Ca, Mg, Mn, P and are characterised by lower ratios of C:P and N:P (p<0.05), which confirms the previously obtained data (Sukhareva, Lukina, 2014), whereas pine needles are characterised by a higher content of C and N (Table 1), which is consistent with the results of other authors (Leonard et al., 2020). At the same time, it should be noted that, unlike the previous results, the concentrations of K in this study turned out to be higher in perennial spruce needles. Higher nitrogen content in pine needles in the last years of life (7–8 years) compared with the spruce needles aged 11–13 years was explained earlier (Sukhareva, Lukina, 2014) by age differences: N, like K and P, refers to mobile nutrition elements (Helmisaari, 1990; Rautio, 1998), that is, translocation of its compounds into younger tissues and depletion of older needles occurs. On the contrary, the content of other mobile nutrients, which belong to the elements of mineral nutrition, namely K, P, and Mg (elements of medium mobility), turned out to be higher in spruce needles. The content of non-mobile elements of mineral nutrition, which include Ca and Mn, as expected, is significantly higher in ageing spruce needles.
Table 1. Chemical composition of living and dead perennial parts of plants in North Taiga pine and spruce forests
| Parameter | Average | Standard deviation | General average | General standard deviation | p | n | ||||
| S-BGC | P-BGC | S-BGC | P-BGC | S-BGC | P-BGC | |||||
| Spruce and pine needles in the last years of life* | ||||||||||
| Ca | mg/kg | 13 878 | 4077 | 3904 | 941 | 11 321 | 5497 | 0 | 51 | 18 |
| Mg | 674 | 495 | 249 | 112 | 628 | 235 | 0.01 | 51 | 18 | |
| K | 3527 | 3212 | 604 | 340 | 3445 | 563 | 0.04 | 51 | 18 | |
| Mn | 2149 | 977 | 923 | 295 | 1844 | 958 | 0 | 51 | 18 | |
| P | 1139 | 928 | 243 | 104 | 1084 | 234 | 0.001 | 51 | 18 | |
| N | 8805 | 10 162 | 1248 | 1579 | 9159 | 1459 | 0.001 | 51 | 18 | |
| Corg | % | 52 | 56 | 4 | 6 | 53 | 5 | 0.01 | 51 | 18 |
| C:N | 61 | 56 | 10 | 8 | 59 | 10 | 0.07 | 51 | 18 | |
| C:P | 478 | 607 | 97 | 96 | 511 | 112 | 0 | 51 | 18 | |
| N:P | 8 | 11 | 2 | 2 | 9 | 2 | 0 | 51 | 18 | |
| Lingonberry — perennial leaves (living parts) | ||||||||||
| Ca | mg/kg | 6535 | 6878 | 1420 | 1063 | 6699 | 1260 | 0.37 | 23 | 21 |
| Mg | 1291 | 1089 | 306 | 127 | 1195 | 257 | 0.01 | 23 | 21 | |
| K | 3917 | 3629 | 482 | 507 | 3780 | 509 | 0.06 | 23 | 21 | |
| Mn | 2091 | 1704 | 371 | 426 | 1907 | 439 | 0.004 | 23 | 21 | |
| P | 952 | 725 | 204 | 81 | 844 | 194 | 0.0001 | 23 | 21 | |
| N | 8832 | 8397 | 1716 | 2275 | 8625 | 1991 | 0.47 | 23 | 21 | |
| Corg | % | 51 | 55 | 3 | 3 | 53 | 4 | 0.001 | 23 | 21 |
| C:N | 60 | 69 | 10 | 17 | 64 | 14 | 0.03 | 23 | 21 | |
| C:P | 556 | 760 | 106 | 85 | 653 | 140 | 0 | 23 | 21 | |
| N:P | 9 | 12 | 1 | 3 | 10 | 2 | 0.002 | 23 | 21 | |
| Crowberry — perennial leaves (living parts) | ||||||||||
| Ca | mg/kg | 8167 | 8755 | 1285 | 1455 | 8342 | 1345 | 0.22 | 26 | 11 |
| Mg | 2381 | 2710 | 273 | 300 | 2479 | 317 | 0.004 | 26 | 11 | |
| K | 4571 | 3823 | 1279 | 593 | 4349 | 1163 | 0.07 | 26 | 11 | |
| Mn | 1016 | 995 | 464 | 187 | 1009 | 399 | 0.88 | 26 | 11 | |
| P | 959 | 1038 | 180 | 113 | 982 | 166 | 0.19 | 26 | 11 | |
| N | 9610 | 10 895 | 2388 | 2900 | 9992 | 2579 | 0.17 | 26 | 11 | |
| Corg | % | 57 | 58 | 3 | 1 | 57 | 3 | 0.64 | 26 | 11 |
| C:N | 63 | 56 | 16 | 15 | 61 | 16 | 0.23 | 26 | 11 | |
| C:P | 613 | 561 | 109 | 63 | 597 | 100 | 0.14 | 26 | 11 | |
| N:P | 10 | 11 | 2 | 3 | 10 | 2 | 0.66 | 26 | 11 | |
| Crowberry — perennial leaves (dead parts) | ||||||||||
| Ca | mg/kg | 8132 | 8453 | 605 | 847 | 8315 | 744 | 0.43 | 6 | 8 |
| Mg | 1950 | 1774 | 332 | 86 | 1850 | 234 | 0.16 | 6 | 8 | |
| K | 3106 | 2034 | 1296 | 293 | 2493 | 998 | 0.05 | 6 | 8 | |
| Mn | 1212 | 678 | 293 | 49 | 907 | 331 | 0.003 | 6 | 8 | |
| P | 720 | 1020 | 175 | 22 | 891 | 189 | 0.003 | 6 | 8 | |
| N | 7577 | 9204 | 2685 | 825 | 8507 | 1959 | 0.12 | 6 | 8 | |
| Corg | % | 57 | 62 | 5 | 5 | 60 | 5 | 0.08 | 6 | 8 |
| C:N | 81 | 69 | 19 | 10 | 74 | 15 | 0.14 | 6 | 8 | |
| C:P | 825 | 613 | 161 | 49 | 704 | 152 | 0.01 | 6 | 8 | |
| N:P | 10 | 9 | 1 | 1 | 10 | 1 | 0.04 | 6 | 8 | |
| Mosses — living parts | ||||||||||
| Ca | mg/kg | 2519 | 2315 | 543 | 292 | 2481 | 508 | 0.38 | 26 | 6 |
| Mg | 974 | 743 | 502 | 80 | 931 | 461 | 0.27 | 26 | 6 | |
| K | 5160 | 4280 | 1136 | 776 | 4995 | 1122 | 0.08 | 26 | 6 | |
| Mn | 558 | 483 | 266 | 128 | 544 | 246 | 0.50 | 26 | 6 | |
| P | 1168 | 852 | 268 | 121 | 1109 | 275 | 0.01 | 26 | 6 | |
| N | 7338 | 5784 | 2013 | 728 | 7046 | 1932 | 0.08 | 26 | 6 | |
| Corg | % | 45 | 51 | 2 | 4 | 46 | 4 | 0.0001 | 26 | 6 |
| C:N | 65 | 91 | 18 | 18 | 70 | 20 | 0.01 | 26 | 6 | |
| C:P | 400 | 616 | 89 | 120 | 440 | 127 | 0.0002 | 26 | 6 | |
| N:P | 6 | 7 | 1 | 1 | 6 | 1 | 0.33 | 26 | 6 | |
| Mosses — dead parts | ||||||||||
| Ca | mg/kg | 4288 | 4456 | 1129 | 1095 | 4384 | 1069 | 0.77 | 6 | 8 |
| Mg | 668 | 1884 | 139 | 788 | 1363 | 855 | 0.01 | 6 | 8 | |
| K | 3434 | 2645 | 855 | 887 | 2983 | 932 | 0.12 | 6 | 8 | |
| Mn | 922 | 615 | 302 | 135 | 747 | 264 | 0.03 | 6 | 8 | |
| P | 952 | 898 | 135 | 122 | 922 | 126 | 0.43 | 6 | 8 | |
| N | 6656 | 8600 | 497 | 2602 | 7767 | 2177 | 0.10 | 6 | 8 | |
| Corg | % | 45 | 51 | 4 | 8 | 49 | 7 | 0.14 | 6 | 8 |
| C:N | 69 | 62 | 8 | 10 | 65 | 9 | 0.17 | 6 | 8 | |
| C:P | 485 | 566 | 82 | 39 | 531 | 72 | 0.04 | 6 | 8 | |
| N:P | 7 | 9 | 2 | 2 | 8 | 2 | 0.04 | 6 | 8 | |
Note: S-BGC— spruce biogeocenosis; P-BGC — pine biogeocenosis; * — data on the composition of spruce needles are presented in S-BGC, pine needles in C-BGTS; p — probability of Type I error when calculating the V-test
Living perennial lingonberry leaves in spruce forests, unlike leaves in pine forests, are characterised by significantly lower ratios of elements (C:N, C:P, N:P) and a higher content of nutrients (Mg, Mn, P), which is consistent with data on the composition of leaves current year (Isaeva, Sukhareva, 2013). However, the carbon content is higher in perennial lingonberry leaves in pine forests (p<0.05).
The litterfall and material of perennial living organs of crowberry and mosses showed distinct differences in chemical composition depending on the type of forest only in relation to Mn and C: the content of manganese is higher in spruce forests, while carbon content is higher in pine forests. It is noteworthy that live ageing pine needles also contain more carbon than spruce needles, which turned out to be rich in manganese. It is known that the assimilating organs of Scots pine show a higher intensity of photosynthesis processes compared to spruce (Tuzhilkina, 1984; Suvorova, 2006; Molchanov, 2020; Yang et al., 2020). A higher carbon content in the plant tissues of shrubs and mosses in pine forests may be associated with a higher intensity of photosynthesis of plants under the canopy of pine trees: dense low-hanging crowns of spruce intercept solar radiation much more effectively than high-raised sparse crowns of Scots pine (Kishchenko, 2019). Accordingly, with better illumination and less closeness of the crowns, plants of the ground cover produce organic matter more efficiently and accumulate more carbon in tissues (Atkina, 2000; Zubkova et al., 2022). In spruce forests, the increased Mn content in ground cover plants may be due to the effect of the litterfall of spruce needles rich in this nutrient on its content in soils.
The content of the other nutrients did not show clear dependencies on the type of forest. In the dead leaves of the crowberry, the K content is also higher in spruce forests, but the P content is higher in pine forests. According to the data obtained earlier at these research sites, the leaves of this year’s crowberry are characterised by a higher content of K, Mn, and P in lichen-shrub pine forests compared with shrub-green-moss spruce forests (Isaeva, Sukhareva, 2013). The ratios of elements in the long-term dead leaves of the crowberry also showed differences: the C:P ratio was lower in spruce forests, and N:P was lower in pine forests. Green mosses (living perennial parts) in spruce forests are richer in phosphorus and in pine forests in carbon, which is consistent with the literature data on the composition of green mosses (Sukhareva, 2018). At the same time, the C:N and C:P ratios are lower in spruce forests. The dead parts of mosses were characterised by differences in the content of other elements: Mg is higher in pine forests, and Mn is higher in spruce forests; the C:P and N:P ratios are lower in spruce forests.
Thus, the content of nutrients and carbon in the ageing and dead leaves of shrubs, as well as in the tissues of mosses, varies depending on the type of forest, which is most clearly manifested in relation to Mn and Corg: in spruce forests, the plant material of shrubs and mosses is enriched with manganese; in pine forests, with carbon. This can be explained by differences in the growing conditions formed by the dominant woody plants: the fertility and soil moisture of spruce forests is higher than that of pine forests, under the same climatic conditions and on the same soil-forming rocks (Lukina et al., 2008). However, in pine forests, due to the sparsity of the crowns, more favourable lighting conditions are created for active photosynthesis of ground cover plants and, accordingly, more active carbon accumulation. It is also likely that the chemical composition of the ground cover plants is directly related to the composition of the litterfall of assimilating organs of woody edifier plants: the increased Mn content in the plants of the ground cover is influenced by its entry into the soil with the litterfall of spruce needles rich in manganese.
Rate of decomposition and changes in the chemical composition of plant residues during the destruction process
Differences in the rate of mass loss may be due to the initial quality and environmental conditions. According to the mass loss rate after the first year of decomposition, the litterfall of the studied species is distributed as follows: lingonberry > spruce needles > crowberry > mosses; after the second year: spruce needles > crowberry > lingonberry > mosses. In pine biogeocenoses, the decomposition rate ranges are as follows: after the first year: crowberry > lingonberry > pine needles > mosses; after the second year: pine needles > lingonberry > crowberry > mosses (Table 2). As in an earlier study conducted in spruce forests, with a relatively high initial value of the C:N ratio in moss litterfall in both spruce and pine forests, the rate of loss of their mass was lower than in other species (Lukina et al., 2017). Other studies have also shown that moss litterfall decomposes slowly (Wardle et al., 2003; Cornelissen et al., 2007; Hilli, 2013), which may be due to the high content of unidentified phenolic compounds in the cell walls of mosses and a wide C:N ratio in plant residues (Ligrone et al., 2008; Lukina et al., 2017).
Table 2. Loss of mass, nutrients, and lignin (in %), and stoichiometric ratios of elements during decomposition of active fractions of plant litterfall in North Taiga pine and spruce forests
| Parameter | S-BGC | P-BGC | p | n | |||
| Average | Standard error | Average | Standard error | S-BGC | P-BGC | ||
| 1 year | |||||||
| Needles* | |||||||
| Mass loss | 20 | 1 | 16 | 1 | 0.01 | 5 | 5 |
| Ca | 12 | 1 | 8 | 8 | 1.00 | 4 | 4 |
| Mg | 15 | 3 | 12 | 1 | 0.19 | 4 | 4 |
| K | 45 | 1 | 24 | 2 | 0.03 | 4 | 4 |
| Mn | –33 | 7 | 1 | 1 | 0.03 | 4 | 4 |
| P | –32 | 2 | –8 | 2 | 0.03 | 4 | 4 |
| N | 4 | 2 | –2 | 7 | 0.47 | 4 | 4 |
| Corg | 15 | 3 | 12 | 3 | 0.47 | 4 | 4 |
| Lignin | –80 | 4 | –10 | 3 | 0.03 | 4 | 4 |
| C:N | 48 | 3 | 99 | 8 | 0.03 | 4 | 4 |
| C:P | 538 | 20 | 1365 | 38 | 0.03 | 4 | 4 |
| N:P | 11 | 0.3 | 14 | 1 | 0.03 | 4 | 4 |
| Lignin:N | 32 | 0.4 | 45 | 3 | 0.03 | 4 | 4 |
| Lingonberry | |||||||
| Mass loss | 20 | 1 | 16 | 0.3 | 0.01 | 5 | 5 |
| Ca | 5 | 0.1 | 10 | 4 | 0.31 | 4 | 4 |
| Mg | 5 | 4 | 16 | 1 | 0.03 | 4 | 4 |
| K | 36 | 4 | 35 | 2 | 0.67 | 4 | 4 |
| Mn | 8 | 5 | –2 | 2 | 0.31 | 4 | 4 |
| P | –4 | 7 | 8 | 1 | 0.31 | 4 | 4 |
| N | 2 | 3 | –3 | 1 | 0.31 | 4 | 4 |
| Corg | 14 | 6 | 7 | 4 | 0.67 | 4 | 4 |
| Lignin | –172 | 6 | –193 | 16 | 0.67 | 4 | 4 |
| C:N | 44 | 2 | 61 | 5 | 0.03 | 4 | 4 |
| C:P | 578 | 78 | 693 | 42 | 0.67 | 4 | 4 |
| N:P | 13 | 1 | 11 | 0.4 | 0.67 | 4 | 4 |
| Lignin:N | 42 | 0.4 | 52 | 3 | 0.03 | 4 | 4 |
| Crowberry | |||||||
| Mass loss | 20 | 3 | 17 | 2 | 0.21 | 5 | 5 |
| Ca | 14 | 6 | 6 | 2 | 0.67 | 4 | 4 |
| Mg | 21 | 0.3 | 29 | 2 | 0.03 | 4 | 4 |
| K | 41 | 4 | 53 | 3 | 0.03 | 4 | 4 |
| Mn | 10 | 5 | 9 | 3 | 0.89 | 4 | 4 |
| P | 9 | 8 | 13 | 4 | 0.89 | 4 | 4 |
| N | 10 | 3 | 5 | 6 | 0.31 | 4 | 4 |
| Corg | 28 | 4 | 12 | 4 | 0.03 | 4 | 4 |
| Lignin | 7 | 3 | 6 | 1 | 0.89 | 4 | 4 |
| C:N | 40 | 1 | 62 | 7 | 0.03 | 4 | 4 |
| C:P | 499 | 14 | 626 | 59 | 0.11 | 4 | 4 |
| N:P | 12 | 1 | 10 | 0.4 | 0.03 | 4 | 4 |
| Lignin:N | 30 | 0.01 | 39 | 5 | 0.03 | 4 | 4 |
| Mosses | |||||||
| Mass loss | 4 | 1 | 10 | 0.4 | 0.01 | 5 | 5 |
| Ca | –7 | 3 | 8 | 10 | 0.31 | 4 | 4 |
| Mg | 11 | 7 | 32 | 12 | 0.31 | 4 | 4 |
| K | 43 | 4 | 54 | 8 | 0.67 | 4 | 4 |
| Mn | 1 | 9 | 35 | 6 | 0.11 | 4 | 4 |
| P | 5 | 4 | 28 | 4 | 0.03 | 4 | 4 |
| N | –11 | 5 | 27 | 7 | 0.03 | 4 | 4 |
| Corg | –3 | 7 | 12 | 4 | 0.11 | 4 | 4 |
| Lignin | 2 | 3 | 4 | 6 | 0.89 | 4 | 4 |
| C:N | 69 | 4 | 77 | 0.3 | 0.19 | 4 | 4 |
| C:P | 623 | 35 | 684 | 42 | 0.47 | 4 | 4 |
| N:P | 9 | 0.2 | 9 | 1 | 0.47 | 4 | 4 |
| Lignin:N | 26 | 1 | 34 | 3 | 0.03 | 4 | 4 |
| Year 2 | |||||||
| Needles* | |||||||
| Mass loss | 37 | 1 | 29 | 0.5 | 0.01 | 5 | 5 |
| Ca | 15 | 5 | 4 | 1 | 0.06 | 4 | 4 |
| Mg | 20 | 5 | 29 | 3 | 0.19 | 4 | 4 |
| K | 80 | 1 | 50 | 1 | 0.03 | 4 | 4 |
| Mn | –11 | 14 | 16 | 3 | 0.31 | 4 | 4 |
| P | 10 | 14 | –1 | 2 | 0.89 | 4 | 4 |
| N | 11 | 2 | 19 | 2 | 0.06 | 4 | 4 |
| Corg | 28 | 4 | 27 | 3 | 0.89 | 4 | 4 |
| Lignin | –35 | 9 | 5 | 1 | 0.03 | 4 | 4 |
| C:N | 44 | 3 | 103 | 7 | 0.03 | 4 | 4 |
| C:P | 711 | 91 | 1215 | 39 | 0.03 | 4 | 4 |
| N:P | 17 | 3 | 12 | 0.4 | 0.31 | 4 | 4 |
| Lignin:N | 26 | 2 | 49 | 2 | 0.03 | 4 | 4 |
| Lingonberry | |||||||
| Mass loss | 33 | 3 | 26 | 1 | 0.21 | 5 | 5 |
| Ca | 6 | 0.2 | 9 | 1 | 0.03 | 4 | 4 |
| Mg | 9 | 3 | 30 | 1 | 0.03 | 4 | 4 |
| K | 62 | 1 | 74 | 1 | 0.03 | 4 | 4 |
| Mn | 5 | 2 | 2 | 1 | 0.31 | 4 | 4 |
| P | 4 | 1 | 27 | 2 | 0.03 | 4 | 4 |
| N | 4 | 1 | 6 | 3 | 0.67 | 4 | 4 |
| Corg | 28 | 6 | 19 | 3 | 0.67 | 4 | 4 |
| Lignin | –131 | 27 | –185 | 17 | 0.31 | 4 | 4 |
| C:N | 38 | 3 | 59 | 3 | 0.03 | 4 | 4 |
| C:P | 510 | 47 | 772 | 31 | 0.03 | 4 | 4 |
| N:P | 13 | 0.2 | 13 | 1 | 0.89 | 4 | 4 |
| Lignin:N | 36 | 4 | 55 | 5 | 0.03 | 4 | 4 |
| Crowberry | |||||||
| Mass loss | 34 | 3 | 25 | 2 | 0.06 | 5 | 5 |
| Ca | 26 | 6 | 16 | 4 | 0.31 | 4 | 4 |
| Mg | 42 | 3 | 40 | 3 | 0.67 | 4 | 4 |
| K | 61 | 3 | 72 | 2 | 0.11 | 4 | 4 |
| Mn | 14 | 1 | 17 | 2 | 0.31 | 4 | 4 |
| P | 20 | 3 | 30 | 3 | 0.03 | 4 | 4 |
| N | 14 | 2 | 0.3 | 2 | 0.03 | 4 | 4 |
| Corg | 40 | 4 | 28 | 3 | 0.11 | 4 | 4 |
| Lignin | 22 | 3 | 13 | 0.3 | 0.03 | 4 | 4 |
| C:N | 35 | 2 | 48 | 2 | 0.03 | 4 | 4 |
| C:P | 459 | 18 | 644 | 40 | 0.03 | 4 | 4 |
| N:P | 13 | 0.1 | 13 | 0.3 | 0.89 | 4 | 4 |
| Lignin:N | 26 | 0.3 | 34 | 2 | 0.03 | 4 | 4 |
| Mosses | |||||||
| Mass loss | 9 | 2 | 14 | 1 | 0.04 | 5 | 5 |
| Ca | –18 | 2 | 13 | 9 | 0.03 | 4 | 4 |
| Mg | 20 | 8 | 37 | 12 | 0.67 | 4 | 4 |
| K | 62 | 4 | 71 | 3 | 0.06 | 4 | 4 |
| Mn | –19 | 1 | 33 | 11 | 0.03 | 4 | 4 |
| P | 11 | 2 | 37 | 3 | 0.03 | 4 | 4 |
| N | –6 | 1 | 16 | 17 | 0.89 | 4 | 4 |
| Corg | –2 | 10 | 23 | 7 | 0.06 | 4 | 4 |
| Lignin | 21 | 7 | 14 | 5 | 0.31 | 4 | 4 |
| C:N | 72 | 8 | 61 | 4 | 0.11 | 4 | 4 |
| C:P | 655 | 83 | 678 | 15 | 0.67 | 4 | 4 |
| N:P | 9 | 0.2 | 11 | 1 | 0.03 | 4 | 4 |
| Lignin:N | 22 | 2 | 29 | 4 | 0.31 | 4 | 4 |
Note: S-BGC — spruce biogeocenosis; P-BGC — pine biogeocenosis; * — data on the composition of spruce needles are presented in S-BGC, pine needles in P-BGC; p — probability of Type I error for the Mann-Whitney U-test
Both after the first and after the second year of decomposition, spruce needles significantly lost mass faster than pine needles did (20% vs. 16% and 37% vs. 29%, respectively); more active K losses and accumulation of lignin (p<0.05) were observed (Tables 2, 3). In addition, in the first year of decomposition, spruce needles had demonstrated a more active accumulation of P. The spruce needles had a higher enrichment coefficient for Mn, P, and lignin after the first year, and for Mg, N, and lignin after the second one. The enrichment coefficient for K was higher for pine needles both after the first and after the second year of decomposition. Stoichiometric ratios C:N, C:P and lignin:N after the first and second years of decomposition were lower for the spruce needles litter.
Table 3. Enrichment coefficient of active fractions of plant litterfall in North Taiga pine and spruce forests
| Parameter | S-BGC | P-BGC | p | n | |||
| Average | Standard error | Average | Standard error | S-BGC | P-BGC | ||
| 1 year | |||||||
| Needles* | |||||||
| Ca | 1.10 | 0.01 | 1.09 | 0.08 | 1.00 | 4 | 4 |
| Mg | 1.06 | 0.05 | 1.05 | 0.02 | 1.00 | 4 | 4 |
| K | 0.69 | 0.01 | 0.91 | 0.03 | 0.03 | 4 | 4 |
| Mn | 1.67 | 0.10 | 1.18 | 0.01 | 0.03 | 4 | 4 |
| P | 1.65 | 0.03 | 1.28 | 0.03 | 0.03 | 4 | 4 |
| N | 1.20 | 0.02 | 1.22 | 0.08 | 0.89 | 4 | 4 |
| Corg | 1.06 | 0.04 | 1.04 | 0.04 | 0.67 | 4 | 4 |
| Lignin | 2.27 | 0.03 | 1.31 | 0.04 | 0.03 | 4 | 4 |
| Lingonberry | |||||||
| Ca | 1.19 | 0.02 | 1.07 | 0.04 | 0.11 | 4 | 4 |
| Mg | 1.18 | 0.07 | 1.00 | 0.005 | 0.03 | 4 | 4 |
| K | 0.80 | 0.06 | 0.78 | 0.03 | 0.89 | 4 | 4 |
| Mn | 1.16 | 0.08 | 1.21 | 0.02 | 0.89 | 4 | 4 |
| P | 1.30 | 0.10 | 1.10 | 0.01 | 0.11 | 4 | 4 |
| N | 1.22 | 0.02 | 1.23 | 0.02 | 0.31 | 4 | 4 |
| Corg | 1.07 | 0.06 | 1.11 | 0.05 | 0.67 | 4 | 4 |
| Lignin | 3.39 | 0.02 | 3.49 | 0.18 | 0.89 | 4 | 4 |
| Crowberry | |||||||
| Ca | 1.06 | 0.04 | 1.13 | 0.04 | 0.67 | 4 | 4 |
| Mg | 0.99 | 0.03 | 0.86 | 0.03 | 0.03 | 4 | 4 |
| K | 0.73 | 0.03 | 0.57 | 0.04 | 0.03 | 4 | 4 |
| Mn | 1.11 | 0.03 | 1.09 | 0.05 | 0.67 | 4 | 4 |
| P | 1.12 | 0.06 | 1.05 | 0.06 | 0.31 | 4 | 4 |
| N | 1.11 | 0.00 | 1.15 | 0.08 | 0.31 | 4 | 4 |
| Corg | 0.89 | 0.02 | 1.05 | 0.04 | 0.03 | 4 | 4 |
| Lignin | 1.15 | 0.00 | 1.13 | 0.03 | 0.31 | 4 | 4 |
| Mosses | |||||||
| Ca | 1.12 | 0.03 | 1.02 | 0.10 | 1.00 | 4 | 4 |
| Mg | 0.94 | 0.08 | 0.75 | 0.13 | 0.31 | 4 | 4 |
| K | 0.60 | 0.05 | 0.51 | 0.09 | 0.67 | 4 | 4 |
| Mn | 1.04 | 0.10 | 0.72 | 0.07 | 0.11 | 4 | 4 |
| P | 0.99 | 0.03 | 0.80 | 0.04 | 0.03 | 4 | 4 |
| N | 1.17 | 0.05 | 0.81 | 0.07 | 0.03 | 4 | 4 |
| Corg | 1.07 | 0.06 | 0.98 | 0.05 | 0.67 | 4 | 4 |
| Lignin | 1.03 | 0.04 | 1.06 | 0.06 | 0.89 | 4 | 4 |
| Year 2 | |||||||
| Needles* | |||||||
| Ca | 1.35 | 0.07 | 1.36 | 0.02 | 0.89 | 4 | 4 |
| Mg | 1.28 | 0.07 | 1.00 | 0.03 | 0.03 | 4 | 4 |
| K | 0.32 | 0.01 | 0.70 | 0.02 | 0.03 | 4 | 4 |
| Mn | 1.78 | 0.23 | 1.18 | 0.03 | 0.11 | 4 | 4 |
| P | 1.43 | 0.23 | 1.42 | 0.03 | 0.89 | 4 | 4 |
| N | 1.41 | 0.02 | 1.14 | 0.03 | 0.03 | 4 | 4 |
| Corg | 1.15 | 0.06 | 1.03 | 0.03 | 0.31 | 4 | 4 |
| Lignin | 2.16 | 0.14 | 1.34 | 0.02 | 0.03 | 4 | 4 |
| Lingonberry | |||||||
| Ca | 1.38 | 0.06 | 1.23 | 0.02 | 0.11 | 4 | 4 |
| Mg | 1.33 | 0.02 | 0.95 | 0.02 | 0.03 | 4 | 4 |
| K | 0.55 | 0.01 | 0.35 | 0.02 | 0.03 | 4 | 4 |
| Mn | 1.40 | 0.09 | 1.33 | 0.03 | 0.89 | 4 | 4 |
| P | 1.41 | 0.07 | 0.99 | 0.03 | 0.03 | 4 | 4 |
| N | 1.40 | 0.05 | 1.28 | 0.06 | 0.31 | 4 | 4 |
| Corg | 1.04 | 0.04 | 1.11 | 0.04 | 0.31 | 4 | 4 |
| Lignin | 3.34 | 0.24 | 3.86 | 0.20 | 0.31 | 4 | 4 |
| Crowberry | |||||||
| Ca | 1.10 | 0.04 | 1.12 | 0.03 | 0.67 | 4 | 4 |
| Mg | 0.87 | 0.01 | 0.80 | 0.03 | 0.11 | 4 | 4 |
| K | 0.59 | 0.02 | 0.38 | 0.02 | 0.03 | 4 | 4 |
| Mn | 1.30 | 0.03 | 1.11 | 0.04 | 0.03 | 4 | 4 |
| P | 1.21 | 0.01 | 0.93 | 0.06 | 0.03 | 4 | 4 |
| N | 1.30 | 0.02 | 1.34 | 0.05 | 0.67 | 4 | 4 |
| Corg | 0.89 | 0.03 | 0.97 | 0.06 | 0.67 | 4 | 4 |
| Lignin | 1.17 | 0.00 | 1.17 | 0.03 | 0.89 | 4 | 4 |
| Mosses | |||||||
| Ca | 1.33 | 0.02 | 1.01 | 0.10 | 0.03 | 4 | 4 |
| Mg | 0.90 | 0.09 | 0.74 | 0.14 | 0.89 | 4 | 4 |
| K | 0.42 | 0.04 | 0.34 | 0.04 | 0.31 | 4 | 4 |
| Mn | 1.33 | 0.01 | 0.78 | 0.12 | 0.03 | 4 | 4 |
| P | 1.00 | 0.03 | 0.73 | 0.03 | 0.03 | 4 | 4 |
| N | 1.19 | 0.01 | 0.97 | 0.19 | 0.89 | 4 | 4 |
| Corg | 1.14 | 0.11 | 0.90 | 0.08 | 0.06 | 4 | 4 |
| Lignin | 0.88 | 0.08 | 1.00 | 0.06 | 0.31 | 4 | 4 |
Note: S-BGC— spruce biogeocenosis; P-BGC — pine biogeocenosis; * — data on the composition of spruce needles are presented in S-BGC, pine needles in P-BGC; p — probability of Type I error for the Mann-Whitney U-test
Lingonberry leaf litterfall decomposed significantly faster in the spruce forest in the first year; in the second year the differences leveled out, but differences in the chemical composition of the litterfall became more pronounced: the enrichment coefficients for Mg, K, and P were higher in the spruce forest, and, accordingly, in the pine forest, lingonberry leaves lost more Ca, Mg, K, and P (p<0.05). C:N and lignin:N ratios after the first and second years were lower in the spruce forest. During the study period, the leaves of the crowberry decomposed in spruce and pine forests at comparable rates (Table 2). However, in the first year the enrichment coefficients for Mg and K were higher in the spruce forest, and the losses of Mg and K were higher in the pine forest; conversely, the enrichment coefficient for C was higher in the pine forest, and the losses were higher in the spruce forest. In the second year the enrichment coefficients for K, Mn, and P were higher in the spruce forest, while the litterfall of crowberry leaves during decomposition lost more N and lignin in the spruce forest, and P in the pine forest (p<0.05). The ratios of C:N and lignin:N in the litterfall of crowberry leaves were narrower in the spruce forest throughout the study period.
The differences found for shrubs may indicate more active processes of transformation of the chemical composition of the litterfall in spruce forests compared with pine forests, which can be explained by the initial concentrations of elements. Thus, a higher initial Mn content in spruce needles and ground cover plant tissues and its accumulation during decomposition can accelerate decomposition processes by increasing the content of the Mn peroxidase enzyme responsible for the decomposition of lignin (Orlova et al., 2011). An increased carbon content in plant tissues may indicate a high content of organic substances, such as lignin, resistant to decomposition. According to the results of earlier work, the mixed litterfall of evergreen plants of spruce forests (spruce needles, lingonberry and crowberry leaves) decomposed faster within two years than pine (pine needles, lingonberry and crowberry leaves), which was also associated with differences in the quality of plant residues: a higher content of nutrients and narrower C:N and lignin:N ratios in the litterfall and in the soils of spruce forests (Lukina et al., 2008; Ivanova et al., 2019).
Differences in the activity of decomposition processes in spruce and pine forests may be due not only to the initial quality of plant material. The main destructive organisms in boreal forests are saprotrophic fungi, which decompose litterfall most effectively (Hobbie et al., 1999; Bödeker et al., 2016). According to the literature data, the total biomass of microorganisms, including fungi, is higher in spruce biogeocenoses in comparison with pine ones (Nikonov et al., 2001; Polyanskaya et al., 2001), and the length of the fungal mycelium in organogenic soil horizons is also greater in spruce forests (Evdokimova, Mozgova, 2001). This may be due to the relatively low soil moisture in pine forests (Nikonov et al., 2004).
An important factor that affects the rate of decomposition is soil temperature, which regulates the activity of destructive organisms; in particular, low temperatures limit the decomposition processes (Vorobyova, Naumova, 2009; Rief et al., 2012). However, according to measurements carried out in 2015–2021 using temperature loggers embedded under organogenic soil horizons, the average monthly temperatures in spruce forests were lower than those in pine forests, the difference reached 2 °C (Fig. 1). This leads to the conclusion that the quality of the litterfall may have a more significant effect on the rate of decomposition than the temperature of the soil.
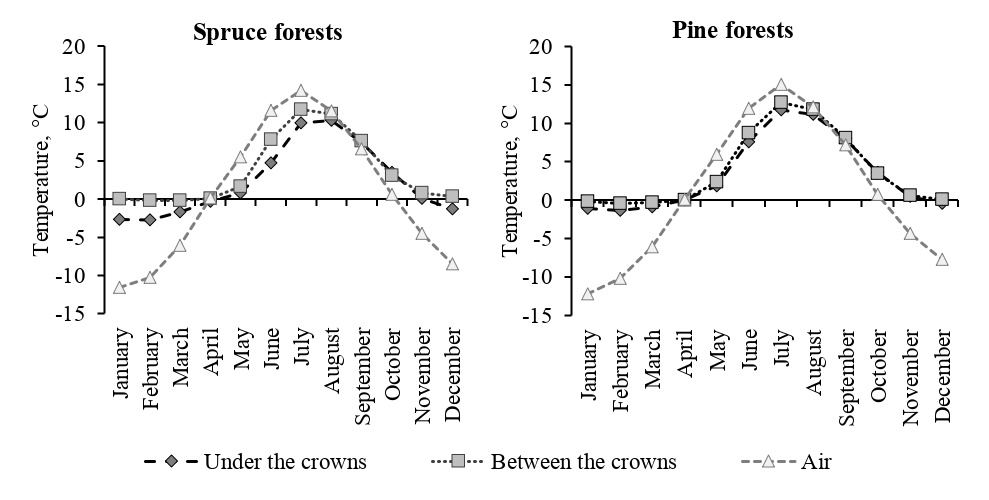
Figure 1. Soil and air temperature in spruce and pine forests, measured in the 2015–2021 using temperature loggers under tree crowns, in intercrown spaces, and on trees
The dead parts of green mosses, despite their higher quality in spruce forests, unlike all other species, decomposed more actively in pine forests over the study period (p<0.05) (Table 2). In the first year the enrichment coefficients for P and N were higher in spruce forests, and losses were higher in pine forests. At the same time, an active accumulation of N by moss residues was observed in the spruce forests. In the second year, green mosses, as well as in the first, lost P more actively in the pine forest. A possible reason may be a higher amount of precipitation in pine forests (32.6 mm versus 26.5 mm in spruce forests) (Ershov, 2021), contributing to the mechanical destruction of moss plant residues and the leaching of mobile elements. In addition, losses of Ca and Mn were observed in the pine forest, and their accumulation was noticed in the spruce forest; enrichment coefficients for Ca, Mn, and P were significantly higher in the spruce forest. After the first year, the lignin:N ratio in the spruce forests was significantly lower; after the second year the N:P ratio was lower (Table 2).
Thus, a combination of factors such as the quality of litterfall, the activity of microorganisms, the temperature conditions, and the amount of precipitation associated with forest type can determine the increased rate of decomposition and the intensity of changes in the chemical composition of plant material in spruce forests compared with pine forests.
CONCLUSION
The results of the study show that the forest type has a significant effect on the rate of decomposition: in spruce forests the rate of decomposition of spruce needles is higher than that of pine needles in pine forests. The quality of plant material and, accordingly, the activity of microorganisms had a significant impact on the rate of decomposition of plant residues in various forest types. Spruce needles are characterised by higher quality: the content of Ca, Mg, K, P, and Mn is higher in ageing spruce needles compared with the content of these elements in pine needles in the last years of life; the C:N and C:P ratios are lower in spruce needles. This explains the more active decomposition processes of spruce litterfall in spruce forests: 37% of the mass loss after two years of decomposition in the spruce forest versus 29% in the pine forest. Ageing, dying organs of shrubs and mosses ready to become litterfall showed ambiguous differences in the content of nutrients depending on the forest type: the quality of living ageing leaves of lingonberry and live parts of mosses was higher in spruce forests. Nevertheless, in the tissues of all plant species in spruce forests, there was more Mn, whereas pine forests had a higher level of C. This may be due to both the habitat conditions that are formed under the action of edifying trees (soil fertility, humidity, and temperature conditions) and the direct influence of woody plants: in pine forests, due to the sparsity of the crowns, more favourable lighting conditions are created for active photosynthesis and carbon accumulation by plants of the ground cover; increased Mn content in ground cover plants of spruce forests is associated with its entry into the soil with the litterfall of spruce needles, which are also rich in manganese. Accordingly, differences in the rate of decomposition of the litterfall of shrubs and mosses between forest types are not clearly manifested. The rate of decomposition of lingonberry leaves was higher in spruce forests after a year of decomposition, which may be due to the higher quality of plant material in the spruce forest: narrow ratios of elements (C:N, C:P, N:P) and high content of nutrients (Mg, Mn, P). At the same time, the dead parts of green mosses decomposed more actively in pine forests, despite their higher quality in spruce forests, which may be explained by the large amount of precipitation in pine forests. Thus, differences in the rate of decomposition of plant residues of needles of woody plants, leaves of shrubs, and shoots of mosses are determined by a combination of factors such as the quality of plant material, temperature conditions, and precipitation associated with the forest type.
ACKNOWLEDGEMENTS
The authors thank Natalia A. Artemkina, Senior Researcher at the Laboratory of Terrestrial Ecosystems of the Institute of North Industrial Ecology Problems of the Federal Research CentreKola Science Centre of the RAS, for analyses of the lignin content in litterfall samples used in decomposition experiments at the monitoring sites of the Institute of North Industrial Ecology Problems.
FINANCING
The study was carried out as part of the work of the youth laboratory of the Centre for Forest Ecology and Productivity of the RAS‘Climate-regulating functions and forest biodiversity’ (registration number 122111500023-6).
REFERENCES
Aponte C., García L. V., Marañón T., Tree species effects on nutrient cycling and soil biota: A feedback mechanism favouring species coexistence, Forest Ecology and Management, 2013, Vol. 309, рр. 36–46.
Atkina L. I., Geografo-lesotipologicheskie zakonomernosti struktury i zapasa napochvennogo pokrova taezhnykh lesov: avtoref. diss. dokt. s-kh. nauk (Geographical and forest typological patterns of the structure and reserve of the ground cover of taiga forests, Doctor’s agricult. sci. thesis), Ekaterinburg, 2000, 37 p.
Atlas Murmanskoi oblasti (Atlas of the Murmansk region), Moscow: Izd-vo Glavnogo Upravleniya Geodezii i Kartografii pri Sovete Ministrov SSSR, 1971, 44 p.
Belov N. P., Baranovskaya A. V., Pochvy Murmanskoi oblasti (Soils of the Murmansk region), Leningrad: Nauka, 1969, 147 p.
Berg B., Litter decomposition and organic matter turnover in northern forest soils, Forest Ecology and Management, 2000, Vol. 133, pp. 13–22.
Berg B., McClaugherty C., Plant litter — decomposition, humus formation, carbon sequestration, 2nd, Germany: Springer-Verlag Berlin Heidelberg, 2008, 340 p.
Bobkova K. S., Rol’ lesnoj podstilki v funkcionirovanii khvojnykh ehkosistem Evropejskogo Severa (The role of forest litter in the functioning of coniferous ecosystems of the European North), Vestnik Instituta biologii Komi NC URO RAN, 2000, No 9 (35), 2000, available at: https://kurl.ru/oMrnF (November 15, 2023)
Bödeker I. T. M., Lindahl B. D., Olson Å., Clemmensen K. E., Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently, Functional Ecology. British Ecological Society, 2016, Vol. 30, No 12, рр. 1967–1978.
Bradford M. A., Berg B., Maynard D. S., Wieder W. R., Wood S. A., Understanding the dominant controls on litter decomposition, Journal of Ecology, 2016, Vol. 104, No 1, рр. 229–238.
Chavez-Vergara B., Merino A., Vázquez-Marrufo G., García-Oliva F., Organic matter dynamics and microbial activity during decomposition of forest floor under two native neotropical oak species in a temperate deciduous forest in Mexico, Geoderma, 2014, Vol. 235-236, рр. 133–145.
Chertov O. G., Men’shikova G. P., Izmenenie lesnykh pochv pod deistviem kislykh osadkov (Changes in forest soils under the influence of acid precipitation), Izvestiya AN SSSR. Seriya biologiya, 1983, No 6, pp. 110–115.
Cornelissen J. H. C., van Bodegom P. M., Aerts R., Callaghan T. V., van Logtestijn R. S. P., …, & Team M. O. L., Global negative vegetation feedback to climate warming responses of leaf decomposition rates in cold biomes, Ecology Letters, 2007, Vol. 10, рр. 619–627.
De Marco A., Vittozzi P., Rutigliano F. A., Virzo de Santo A. Nutrient dynamics during decomposition of four different pine litters [in:] Leone V., Lovreglio R. (Eds.), Proceedings of the international workshop MEDPINE 3: conservation, regeneration and restoration of Mediterranean pines and their ecosystems, Bari: CIHEAM, 2007, pp. 73–77.
Ershov V. V., Fitogennoe var’irovanie sostava atmosfernykh vypadenii i pochvennykh vod severotaezhnykh lesov v usloviyakh aerotekhnogennogo zagryazneniya, Diss. … kand. biol. nauk (Phytogenic variation of the composition of atmospheric precipitation and soil waters of the North Taiga forests under aerotechnogenic pollution, Candidate’s biol. sci. thesis), Apatity, 2021, 188 p.
Evdokimova G. A., Mozgova N. P., Mikroorganizmy tundrovykh i lesnykh podzolov Kol’skogo Severa (Microorganisms of tundra and forest podzols of the Kola North), Apatity: KNTs RAN, 2001, 184 p.
Fang X., Zhao L., Zhou G., Huang W., Liu J., Increased litter input increases litter decomposition and soil respiration but has minor effects on soil organic carbon in subtropical forests, Plant Soil, 2015, Vol. 392, pp. 139–153.
Fedorets N. G., Bakhmet O. N., Osobennosti formirovaniya pochv i pochvennogo pokrova Karelo-Kol’skogo regiona (Peculiarities of soil and soil cover formation in the Karelia — Kola region), Trudy Karel’skogo nauchnogo tsentra RAN, 2016, No 12, pp. 39‒51.
Helmisaari H.-S., Temporal variation in nutrient concentrations of Pinus sylvestris needles, Canadian Journal of Forest Research, 1990, No 5, pp. 177–193.
Hilli S., Significance of litter production of forest stands and ground vegetation in the formation of organic matter and storage of carbon in boreal coniferous forests, Forest Condition Monitoring in Finland — National report, P. Merilä, S. Jortikka (Eds.), The Finnish Forest Research Institute, 2013, pp. 77–83.
Hobbie E. A., Macko S. A., Shugart H. H., Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence, Oecologia, 1999, Vol. 118, No 3, pp. 353–360.
Högberg P., Näsholm T., Franklin O., Högberg M. N., Tamm Review: On the nature of the nitrogen limitation to plant growth in Fennoscandian boreal forests, Forest Ecology and Management, 2017, Vol. 403, pp. 161–185.
Husson F., Le S., Pages J., Exploratory multivariate analysis by example using R, 2nd edition, Chapman & Hall/CRC, 2017, 248 p.
Isaeva L. G., Sukhareva T. A., Elementnyi sostav dikorastushchikh kustarnichkov v zone vozdeistviya kombinata “Severonikel’”: dannye mnogoletnego monitoring (Elemental composition of wild small shrubs in the area of influence of “Severoniсkel” combine: data of long-term monitoring), Tsvetnye metally, 2013, No 10, pp. 87–92.
Ivanova E. A., Lukina N. V., Danilova M. A., Artemkina N. A., Smirnov V. E., Ershov V. V., Isaeva L. G., Vliyanie aerotekhnogennogo zagryazneniya na skorost’ razlozheniya rastitel’nykh ostatkov v sosnovykh lesakh na severnom predele rasprostraneniya (The effect of air pollution on the rate of decomposition of plant litter at the northern limit of pine forests), Lesovedenie, 2019, No 6, pp. 533–546.
Kishchenko I. T., Lesovedenie i lesnaya ekologiya. Uchebnoe posobie dlya bakalavriata i magistratury (Forestry and forest ecology. Textbook for bachelor’s and Master’s degrees), Moscow: Izd-vo Yurait, 2019, 393 p.
Kolmogorova E. Yu., Ufimtsev V. I., Nekotorye osobennosti khimicheskogo sostava opada sosny obyknovennoi, proizrastayushchei v usloviyakh porodnogo otvala (Some peculiarities of the chemical composition of Scotch pine debris, growing under conditions of coal pit), Uspekhi sovremennogo estestvoznaniya, 2018, No 11, Part. 2, pp. 267–272.
Kuznetsov M. A., Osipov A. F., Rastitel’nyi opad kak komponent biologicheskogo krugovorota ugleroda v zabolochennykh khvoinykh soobshchestvakh srednei taigi (Plant litter as a component of the biological carbon cycle of wet coniferous communities in the middle taiga), Vestnik Instituta biologii Komi NTs Ural’skogo otdeleniya RAN, Izd-vo Instituta biologii Komi NTs UrO RAN, 2011, No 9, pp. 10–12.
Kuznetsov M. A., Vliyanie uslovii razlozheniya i sostava opada na kharakteristiki i zapas podstilki v srednetaezhnom chernichno-sfagnovom el’nike (Effect of decomposition conditions and falloff composition on litter reserves and characteristics in a bilberry-sphagnum spruce forest of middle taiga), Lesovedenie, 2010, No 6, pp. 54–60.
Larionova A. A., Kvitkina A. K., Bykhovets S. S., Lopes-de-Gerenyu V. O., Kolyagin Yu. G., Kaganov V. V., Vliyanie azota na mineralizatsiyu i gumifikatsiyu lesnykh opadov v model’nom eksperimente (The contribution of nitrogen to mineralization and humification of forest litter in simulation study), Lesovedenie, 2017, No 2, pp. 128–139.
Leonard L. T., Mikkelson K., Hao Z., Brodie E. L., Williams K. H., Sharp J. O., A comparison of lodgepole and spruce needle chemistry impacts on terrestrial biogeochemical processes during isolated decomposition, PeerJ, 2020, Vol. 8, Article: e9538.
Ligrone R., Carafa A., Duckett J. G., Renzaglia K. S., Ruel K., Immunocytochemical detection of lignin-related epitopes in cell walls in bryophytes and the charalean alga Nitella, Plant Systematics and Evolution, 2008, Vol. 270, pp. 257–272.
Lukina N. V. Polyanskaya L. M., Orlova M. A., Pitatel’nyi rezhim pochv severotaezhnykh lesov (Nutrient regime of soils of the north taiga forests), Moscow: Nauka, 2008, 342 p.
Lukina N. V., Gorbacheva T. T., Nikonov V. V., Lukina M. A., Spatial variability of soil acidity in Al-Fe-humus podzols of northern taiga, Eurasian Soil Science, 2002, Vol. 35, No 2, pp. 144–155.
Lukina N. V., Nikonov V. V., Biogeokhimicheskie tsikly v lesakh Severa v usloviyakh aerotekhnogennogo zagryazneniya (Biogeochemical cycles in the Northern forest ecosystems subjected to air pollution), Apatity: Izd-vo KNTs RAN, 1996, Part 1, 213 p., Part 2, 192 p.
Lukina N. V., Nikonov V. V., Isaeva L. G., Kislotnost’ i pitatel’nyi rezhim pochv elovykh lesov (Acidity and nutrient regime of spruce forest soils), [in:] Korennye elovye lesa: bioraznoobrazie, struktura, funktsii (Native spruce forests: biodiversity, structure, functions), Saint-Petersburg: Nauka, 2006, 298 p.
Lukina N. V., Nikonov V. V., Pitatel’nyi rezhim lesov severnoi taigi: prirodnye i tekhnogennye aspekty (Nutrient status of north taiga forests: natural regularities and pollution-induced changes), Apatity: Izd-vo KNTsRAN, 1998, 316 p.
Lukina N. V., Orlova M. A., Isaeva L. G., Forest soil fertility: the base of relationships between soil and vegetation, Contemporary Problems of Ecology, 2011, Vol. 4, No 7, pp. 725–733.
Lukina N. V., Orlova M. A., Steinnes E., Artemkina N. A., Gorbacheva T. T., Smirnov V. E., Belova E. A., Plodorodie lesnyh pochv kak osnova vzaimosvyazi pochva — rastitel’nost’ (Fertility of forest soils as the basis of the soil-vegetation relationship), Lesovedenie, 2010, No 5, pp. 45–56.
Molchanov A. G., Comparison of eco-physiological indicators of pine and spruce in the Serebryanoborsky experimental forestry, Lesohozyajstvennaya informaciya, 2020, No 1, pp. 115–124.
Nikonov V. V., Lukina N. V., Bezel’ V. S., Bel’skii E. A., Bespalova A. Yu., …, & Yatsenko-Khmelevskaya M. A., Rasseyannye elementy v boreal’nykh lesakh (Scattered elements in boreal forests), Moscow: Nauka, 2004, 616 p.
Nikonov V. V., Lukina N. V., Polyanskaya L. M., Panikova A. N., Osobennosti rasprostraneniya mikroorganizmov v Al-Fe-gumusovykh podzolakh severotaezhnykh elovykh lesov: prirodnye i tekhnogennye aspekty (Features of the microorganisms distribution in Al-Fe-humus podzols under northern taiga spruce forests: natural and technogenic aspects), Mikrobiologiya, 2001, Vol. 70, No 3, pp. 319–328.
Ogden A. E., Schmidt M. G., Litterfall and soil characteristics in canopy gaps occupied by vine maple in a coastal western hemlock forest, Сanadian Journal of Soil Science, 1997, Vol. 77, No 4, pp. 703-711.
Orlova M. A., Lukina N. V., Kamaev I. O., Smirnov V. E., Kravchenko T. V., Mozaichnost’ lesnykh biogeotsenozov i produktivnost’ pochv (Forest ecosystem mosaics and soil fertility), Lesovedenie, 2011, No 6, pp. 39–48.
Pausas J. G., Litter fall and litter decomposition in Pinus sylvestris forests of the eastern Pyrenees, Journal of Vegetation Science, 1997, Vol. 8, pp. 643–650.
Pereverzev V. N., Lesnye pochvy Kol’skogo poluostrova (Forest soils of the Kola Peninsula), Moscow: Nauka, 2004, 232 p.
Polyanskaya L. M., Nikonov V. V., Lukina N. V., Panikova A. N., Zvyagintsev V. G., Mikroorganizmy Al-Fe-gumusovykh podzolov sosnyakov lishainikovykh v usloviyakh aerotekhnogennogo zagryazneniya (Microorganisms of Al-Fe-humus podzols under lichen pine forests affected by aerotechnogenic pollution), Pochvovedenie, 2001, No 2, pp. 215‒226.
Pomogaibin E. A., Pomogaibin A. V., Vliyanie derev’ev roda Juglans L. na tsellyulozorazrushayushchuyu aktivnost’ pochvy v usloviyakh dendrariya botanicheskogo sada Samarskogo universiteta (Juglans L. genus trees influence on cellulolytic soil activity in Samara University Botanical Garden Dendrarium), Samarskii nauchnyi vestnik, 2018, Vol. 7, No 1 (22), pp. 105–109.
Portillo-Estrada M., Pihlatie M., Korhonen J. F. J., Levula J., Frumau A. K. F., Ibrom A., Lembrechts J. J., Morillas L., Horváth L., Jones S. K., Niinemets Ü., Climatic controls on leaf litter decomposition across European forests and grasslands revealed by reciprocal litter transplantation experiments, Biogeosciences, 2016, Vol. 13, pp. 1621–1633.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2017, available at: http://www.R-project.org (November 15, 2023).
Rahman M. M., Tsukamoto J., Rahman M. M., Yoneyama A., Mostafa K. M., Lignin and its effects on litter decomposition in forests ecosystems, Chemistry and Ecology, 2013, Vol. 29, No 6, pp. 540–553.
Rakhleeva A. A., Semenova T. A., Striganova B. R., Terekhova V. A., Dinamika zoomikrobnykh kompleksov pri razlozhenii rastitel’nogo opada v el’nikakh Yuzhnoi taigi (Dynamics of zoomicrobial complexes upon decomposition of plant litter in spruce forests of the southern taiga), Pochvovedenie, 2011, No 1, pp. 44–55.
Rautio P., Huttunen S., Lamppu J. Seasonal foliar chemistry of northern Scots pine under sulphur and heavy metal pollution, Chemosphere, 1998, Vol. 37, No 2, pp. 271–287.
Rief A., Knapp B. A., Seeber J., Palatability of selected alpine plant litters for the decomposer Lumbricus rubellus (Lumbricidae), PLoS One, 2012, Vol. 7, Article: e45345.
Rowland A. P., Roberts J. D., Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre methods, Communications in Soil Science and Plant Analysis, 1994, Vol. 25, No 3–4, pp. 269–277.
Sukhareva T. A., Elementnyi sostav zelenykh mkhov fonovykh i tekhnogennogo narushennykh territorii (The green moss elemental composition of the background and industrially disturbed areas), Uchenye zapiski Petrozavodskogo gosudarstvennogo universiteta, 2018, No 3 (172), pp. 89–96.
Sukhareva T. A., Lukina N. V., Mineral’nyi sostav assimiliruyushchikh organov khvoinykh derev’ev posle snizheniya urovnya atmosfernogo zagryazneniya na Kol’skom poluostrove (Mineral composition of assimilative organs of conifers after reduction of atmospheric pollution in the Kola peninsula), Ekologiya, 2014. No 2, pp. 97–104.
Suvorova G. G., Fotosinteticheskaya aktivnost’ khvoinykh derev’ev v usloviyakh yuga Srednei Sibir, Avtoref. diss. … dokt. biol. nauk (Photosynthetic activity of coniferous trees in the conditions of the South of Central Siberia, Doctor’s agricult. sci. thesis), Irkutsk, 2006, 40 p.
Tsandekova O. L., Dinamika nakopleniya zoly v opade Acer negundo L. v usloviyakh narushennykh poimennykh fitotsenozov (Dynamics of accumulation of ash in litter Acer negundo L. under conditions of disturbed floodplain phytocenoses), Byulleten’ nauki i praktiki, 2018, Vol. 4, No 12, pp. 148‒152.
Tsvetkov V. F., Lesnoi biogeotsenoz (Forest biogeocenosis), Arkhangelsk, 2004, 267 p.
Tu L-h., Hu H-l., Chen G., Peng Y., Xiao Y-l., Hu T-x., Zhang J., Li X-w., Liu L., Tang Y., Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition, PLoS One, 2014, Vol. 9, No 2, pp. 1–9.
Tuzhilkina V. V., Fotosinteticheskaya aktivnost’ sosny i eli v usloviyakh srednei podzony taigi Komi ASS, Diss. … kand. biol. nauk (Photosynthetic activity of pine and spruce in the conditions of the middle taiga subzone of the Komi ASSR, Candidate’s biol. sci. thesis), Syktyvkar, 1984, 148 p.
Vorob’eva I. G., Naumova A. N., Intensivnost’ protsessa destruktsii rastitel’nogo opada v pochvakh sukhikh mestoobitanii (Intensity of waste degradation in dry habitat soils), III international forest soil science conference: Productivity and resistance of forest soils, Proc. Conf., Petrozavodsk, 7‒11 September, 2009, pp. 192–195.
Vorob’eva L. A., Khimicheskii analiz pochv: Uchebnik (Chemical analysis of soils: Textbook), Moscow: MGU, 1998, 272 p.
Wardle D. A., Nilsson M.-C., Zackrisson O., Gallet C., Determinants of litter mixing effects in a Swedish boreal forest, Soil Biology and Biochemistry, 2003, Vol. 35, No 6, pp. 827–835.
Yang Q., Blanco N. E., Hermida-Carrera C., Lehotai N., Hurry V., Strand Å., Two dominant boreal conifers use contrasting mechanisms to reactivate photosynthesis in the spring, Nature Communications, 2020, Vol. 11, Article 128.
Zhang D., Hui D., Luo Y., Zhou G., Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors, Journal of Plant Ecology, 2008, Vol. 1, No 2, pp. 85–93.
Zubkova E. V., Frolov P. V., Bykhovets S. S., Nadporozhskaya M. A., Frolova G. G., Mozaichnost’ tsenopopulyatsii cherniki i brusniki, i dinamika organicheskogo veshchestva pochvy v sosnyakakh Yuzhnogo Podmoskov’ya (Mosaic structure of blueberry and lingonberry cenopopulations and the dynamics of soil organic matter in the southern Moscow region pine forests), Lesovedenie, 2022, No 1, pp. 34–46.
Reviewer: Candidate of Biological Sciences, G. V. Akhmetova





