- DOI 10.31509/2658-607x-202141a19
INFLUENCE OF SINGLE TREES ON FLORISTIC DIVERSITY OF STEPPE MEADOWS AND POPULATION STRUCTURE OF SOME RARE PLANT SPECIES
![]() Original Russian Text © 2020 E.V. Ruchinskaya, A.V. Gornov published in Forest Science Issues Vol. 3, No. 4, pp. 1-25
Original Russian Text © 2020 E.V. Ruchinskaya, A.V. Gornov published in Forest Science Issues Vol. 3, No. 4, pp. 1-25
E.V. Ruchinskaya*, A.V. Gornov
Center for Forest Ecology and Productivity of the Russian Academy of Sciences
Profsoyuznaya st. 84/32 bldg. 14, Moscow 117997, Russian Federation
*E-mail: elena.ruchinskaya@mail.com
Received 01.11.2020
Accepted 14.12.2020
In the zone of broad-leaved forests of the European Russia, steppe meadows have been preserved showing rich floristic composition and making a significant contribution to the biological diversity of the territories. Bryansk oblast is one of the forest regions in Russia where such meadows are found. Here, steppe meadows with high floristic diversity and a large number of rare plant species have survived. Trees from surrounding forest areas are constantly encroaching on these meadows. Most of the young trees die from regular grass fires and economic activity. However, some individuals survive and reach a generative state, becoming relatively resistant to ground fires. The influence of single trees on the floristic diversity of steppe meadows was studied at two levels of living system organization – coenotic and population levels. Polydominant steppe meadows and polydominant steppe meadows with single generative trees were studied at the coenotic level; and coenopopulations of Iris aphylla, Anemone sylvestris, and Anthericum ramosum were studied at the population level. Collecting the material, we used different methods: geobotanical, demographic, and measurements of environmental factors (illumination, slope steepness, and the frequency of grass fires). Polydominant steppe meadows were found to be were preserved in the middle part of steep slopes unsuitable for haymaking and grazing and subjected to infrequent grass fires. These communities have high floristic diversity and stable coenopopulations of model species. Ontogenetic spectra of Anemone sylvestris, Anthericum ramosum, and Iris aphylla are of the complete left-hand type with the maximum number of individuals. Single trees (Quercus robur, Tilia cordata) have controversial influence on the vegetation of polydominant steppe meadows. On the one hand, with the introduction of trees, species diversity of communities increases. This is due to the fact that trees offer resting places and shelter for birds that spread plant diaspores. On the other hand, mature trees shade the herb cover. This leads to cover reduction and occurrence of steppe and dry meadow species, as well as affects their population structure. The ontogenetic spectrum of Anemone sylvestris is still complete, whereas that of Iris aphylla becomes incomplete, and the spectrum of Anthericum ramosum becomes unfinished.
Key words: steppe meadow, single trees, floristic diversity, coenopopulations, ontogenetic spectrum, state of coenopopulations, Anemone sylvestris, Anthericum ramosum, Iris aphylla
Steppe meadows have survived in the zone of broad-leaved forests of the European Russia (Bulohov, 1977, 2001; Bosek, 1980; Skvorcov, 1982; Averinova, 2010; Semenishhenkov, 2010, 2012; Evstigneev et al., 2011; Panasenko et al., 2013, 2015, etc.). These communities, in general, have rich floristic composition and make a significant contribution to the biological diversity of the territories. However, due to human economic activity and grass fires, such coenoses are in danger of extinction (Zelenaja …, 2012; Evstigneev et al., 2018a; Ruchinskaya, 2019). Woody plants from the surrounding forest areas are constantly encroaching on the steppe meadows preserved in the zone of broad-leaved forests. Most of the young trees die from regular grass fires and economic activities such as haymaking, grazing, etc. However, some individuals survive and go into a generative state (Evstigneev et al., 2018a). Mature trees are relatively resistant to ground fires: their renewal buds are located high and the thick crust of the trunk protects the cambium (Serebrjakov, 1962). Single trees affect the growing conditions of other plants in the meadows. It is known that in phytogeneous fields of trees, illumination, air temperature and humidity, soil temperature and humidity, the amount of precipitation penetrating through the crown, quality of litter, concentration of nutrients and other soil characteristics change significantly (Uranov, 1965; Samojlov, 1983; Nikonov et al., 2002; Ipatov, 2007; Zhuravleva et al., 2012; Orlova et al., 2016). In addition, single trees attract animals of different ecological groups, e.g. soil invertebrates, mouse-like rodents, birds, etc. (Manning et al., 2006; Prevedello et al., 2018). On the one hand, these influence the growing conditions of plants, and on the other hand, they participate in the creation of both intracoenotic and intercoenotic flows of diaspores. Therefore, the objective of this work is to consider the influence of single trees on the floristic composition and the state of coenopopulations of some rare plant species in the steppe meadows.
MATERIALS AND METHODS
The research was carried out in the south-east of Bryansk oblast within the Melovitskie Slopes natural monument (Figure 1). The site is located in Komarichsky-Sevsky physiographic region. It consists of elevated loess plains with ravines, gullies, slopes and outcrops of carbonate rocks on the western offsets of the Central Russian Upland. Botanically and geographically, the territory belongs to the Eastern European Province of the European broad-leaved forest region (Rastitel’nost’…, 1980). Komarichsky-Sevsky district has temperate continental climate. The mean annual temperature is 5.4 οС. The duration of the warm season with above-freezing temperatures is 228 days; the growing season with the temperature above + 5°C is 188 days. Mean annual precipitation is 613 mm; the mean precipitation in the warm season is 342 mm (Prirodnoe …, 1975).
The research was carried out at two levels of living system organization, i.e. at the coenotic and population level. Polydominant steppe meadows and polydominant steppe meadows with single generative trees were studied at the coenotic level. At the population level, the objects of the study were coenopopulations of model plant species Iris aphylla L., Anemone sylvestris L., and Anthericum ramosum L. Iris aphylla is a short rhizome rosette spring-flowering summer-green plant (Figure 2, A). It is a geophyte. Anemone sylvestris is a perennial herbaceous spring-flowering summer-green short rhizome plant (Figure 2, B). It is a hemicryptophyte and geophyte. Anthericum ramosum is a perennial herbaceous summer-green summer-flowering short rhizome plant (Figure 3). It is a hemicryptophyte and geophyte. These species were chosen due to the fact that they are rare, endangered and listed in Red Books of many regions (Krasnaja…, 2002, 2004, 2015, 2016, etc.). Moreover, Iris aphylla is listed in the Red Book of Russia (2008).

Figure 1. Location of the Melovitskie Slopes natural monument. The green line represents the boundaries of the research object. Background satellite imagery by Microsoft Bing Maps
The following research methods were used: geobotanical, demographic, statistical, and measurements of habitat factors. Relevés were made on plots of 100 m2 in 11-fold repetition for each community type. A complete floristic list was compiled at each plot. Species participation was evaluated as scores according to the cover-abundance scale proposed by J. Braun-Blanquet (Mirkin et al., 1989). Species richness and species density were used to assess the species diversity of communities. Species richness is the total number of species in a community, which is obtained on the basis of 11 relevés. Species density is the average number of species per unit area. The names of vascular plants were given according to The Plant List international database (http://www.theplantlist.org/). For relevés analysis of communities, ordination was carried out using Detrended Correspondence Analysis (DCA). This method works successfully with heterogeneous data of relevés (Dzhongman et al., 1999). PC-ORD software was used for calculations. Demographic research was based on ontogenesis periodization proposed by T. A. Rabotnov (1950) and supplemented by A.A. Uranov (1975) and other scientists (Cenopopuljacii …, 1988). Ontogenesis is divided into stages that show morphological and functional differences. Individuals belonging to the same ontogenetic state are grouped together: j – juvenile, im – immature, v – virginile, g1 – young generative, g2 – mature generative, g3 – old generative, ss – sub-senile, s – senile. Ontogenetic states of the model species were determined on the basis of publications (Evstigneev et al., 2018; Ruchinskaya, 2019). The state of coenopopulations was estimated using the number, density, and type of the ontogenetic spectrum. Number is the number of individuals in the study area (Chernova, Bylova, 2007). Population density is the average number of individuals per unit area (Odum, 1986; Cenopopuljacii …, 1988). The type of the ontogenetic spectrum was named according to the classification proposed earlier (Zaugol’nova, 1994b). In the meadows and under the trees, the illuminance was measured hourly with a light meter on a cloudless June day from 10 a.m. to 6 p.m. Illuminance in lux was converted to a percentage of the total illuminance, which was measured in the open space. The slope steepness was measured using a Nikon Forestry Pro rangefinder. The frequency of grass fires was determined by the age of shoots of formation in shrubs (Frangula alnus Mill., Corylus avellana L.). These shoots emerge from dormant buds located in the basal part of the shrub. The former aboveground shoots were destroyed due to fire damage (Figure 4).
RESULT AND DISCUSSION
Polydominant steppe meadows were preserved in the middle part of steep slopes hardly suitable for haymaking and grazing (Figure 5). Grass fires mainly occur once every two years. They limit the introduction of woody plants as young tree species are most vulnerable. For instance, seedlings and juvenile oak plants often die during grass fires (Komarov, 1951). As a result, polydominant communities with high species diversity are formed (Table 1; Suppl. materials). These coenoses are unique since they include species that are characteristic of steppe communities: Ajuga genevensis L., Anemone sylvestris, Aster amellus L., Astragalus cicer L., Campanula sibirica L., Prunus cerasus L., Galium tinctorium L., G. verum L. etc. The ecological-coenotic structure is dominated by plants of the dry meadow group, which also includes the above-named steppe plants. Moist meadow (Festuca pratensis Huds., Hypericum maculatum Crantz, Succisa pratensis Moench, Thalictrum lucidum L. и др.), nemoral forest-edge (Brachypodium pinnatum (L.) Beauv., Peucedanum cervaria (L.) Cusson ex Lapeyr, Laserpitium latifolium L., Lathyrus pisiformis L., L. sylvestris L., Pyrethrum corymbosum (L.) Scop.), and nitrophilous forest-edge (Rubus caesius L. and Valeriana officinalis L.) plants are often found as well. Small participation is typical of forest species, i. e. nemoral – Convallaria majalis L., Corylus avellana, Quercus robur L., Viola mirabilis L., boreal – Frangula alnus, and piny – Pteridium aquilinum, Solidago virgaurea L., and Viola collina Besser. Diaspores of forest and forest-edge species are brought here by animals and wind from the neighbouring pine forest. Moist meadow and nitrophilous species are brought from floodplain communities adjacent to the slope.
Table 1. Characteristics of communities on the steppe slopes. Melovitskie Slopes natural monument
| Indicators | Communities | |
| 1 | 2 | |
| Slope angle | ||
| Slope angle, M ± σ | 37 ± 2.4 | 31 ± 2.4 |
| Slope angle range | 33–41 | 28–37 |
| Number of measurements | 21 | 33 |
| Fires | ||
| Fire frequency, M ± σ | 2.3 ± 1.2 | 2.2 ± 1.0 |
| Number of measurements | 52 | 33 |
| Characteristics of the diversity of vascular plant species | ||
| Average number of species per 100 m2, M ± mM | 51 ± 1.2 | 59 ± 1.2 |
| Range of number of species per 100 m2 | 44–56 | 52–66 |
| Number of species on 11 plots of 100 m2 each | 98 | 107 |
| Number and proportion (%) of species of different ecological-coenotic groups | ||
| Dry meadow | 77 (78.6) | 79 (73.8) |
| Moist meadow | 5 (5.1) | 7 (6.5) |
| Nemoral forest | 4 (4.1) | 8 (7.5) |
| Nemoral forest-edge | 6 (6.1) | 6 (5.6) |
| Piny | 3 (3.1) | 3 (2.8) |
| Boreal forest | 1 (1.0) | 1 (1.0) |
| Nitrophilous forest-edge | 2 (2.0) | 3 (2.8) |
Note. M is the arithmetic mean, and σ is the standard deviation. Communities: 1 – polydominant steppe meadows, 2 – polydominant steppe meadows with single generative trees
Iris aphylla is one of the predominant species in the herb layer of polydominant steppe meadows of the Melovitskie Slopes. Population density of Iris aphylla is 82 plants per 1 m2. The ontogenetic spectrum is complete single-peak with a maximum at v and gн plants (Figure 6, 1a). Iris aphylla is well adapted to high illuminance of open spaces due to the structure of its leaves: they are flattened laterally and vertically oriented (Evstigneev et al., 2018b). Seed renewal of Iris aphylla is facilitated by the activity of animals such as ants and mouse-like rodents that inhabit the slopes and create disturbances. These microsites are characterized by sparse herb cover, loosened substrate, increased aeration and soil temperature, and significant microbiological activity (Zrjanin, 2003; Dauber, Wolters, 2000; Kostrakiewicz, 2004, etc.). For example, a population locus consisting of 10 juvenile plants was found in a 0.03 m2 earth ejections of the mouse-like rodent. The spread of Iris aphylla diaspores is facilitated by ants (Figure 7). R.E. Levina (1957) states that fresh seeds attract these animals with sweet, sticky liquid that is contained in the shell. Our observations showed that ants also spread dry seeds (Evstigneev et al., 2018b).
Anthericum ramosum is a codominant species in the herb layer of steppe meadows. Population density of Anthericum ramosum is 56 plants per 1 m2. Ontogenetic spectrum is complete, left-hand type, single-peak with a maximum at v and gн plants (Figure 6, 2а). The formation of the maximum at v and gн plants is determined by the following: 1) short duration of j and im states; 2) recruitment of plants resting from flowering; 3) recruitment of v with plants of vegetative origin formed as a result of disintegration of g2 plants.

Figure 2. Model plants species in steppe meadows of the Melovitskie Slopes natural monument: А – Iris aphylla, B – Anemone sylvestris. Photo by A.V. Gornov

Figure 3. Model plants species in steppe meadows of the Melovitskie Slopes natural monument: А, B – Anthericum ramosum. Photo by E.V. Ruchinskaya

Figure 4. Coppice shoots of burnt sour cherry (Prunus cerasus). A – general appearance of the shrub, B – base of the shrub. 1 – stump of a burnt perennial shoot, 2 – dead burnt biennial coppice shoot, 3 – live annual shoot that woke up from a dormant bud after a ground fire in the spring (from: Ruchinskaya, 2019)

Figure 5. Polydominant steppe meadows in the territory of the Melovitskie Slopes natural monument. Photo by A.V. Gornov

Figure 6. Ontogenetic spectrum of coenopopulations of model plant species in steppe meadows. 1 – Iris aphylla, 2 – Anthericum ramosum, 3 – Anemone sylvestris. The X axis shows ontogenetic states, and the Y axis – the proportion of individual plants, %. Circled is population density (the number of plants per 1 m2) is shown. Communities: а – polydominant steppe meadows, b – polydominant steppe meadows with single generative trees. Ontogenetic states of trees: j – juvenile, im – immature, v – virginile, gн – temporarily not flowering generative plant, g1 – young generative, g2 – mature generative, g3 – old generative, ss – subsenile, s – senile

Figure 7. Dispersal of fresh Iris aphylla seeds by the red forest ant (Formica rufa). Photo by E.V. Ruchinskaya
Anemone sylvestris can be both an assectator and a codominant in the herb layer of steppe meadows. Population density of A. sylvestris is 75 plants per 1 m2. Ontogenetic spectrum is complete left-hand type with a maximum at im plants (Figure 6, 3a), the density of which is 27 trees per 1 m2. No A. sylvestris individuals of seed origin was found in the studied community. Therefore, the spectrum can be called vegetative and complete. High population density and the complete ontogenetic spectrum are determined by the biology of A. sylvestris. Large number of plants of the pregenerative state is due to the ability of A. sylvestris to vegetative reproduction with deep rejuvenation (Figure 8). Vegetative individuals develop from buds that appear on horizontal adventitious roots (Starostenkova, 1986; Barykina, Potapova, 1994). The beginning of the growth season in early spring, before the herb layer rises, contributes to the accumulation of a sufficient amount of macronutrients required for the formation of generative organs in plans. It is worth noting that the absence of seed individuals is evidence of adverse conditions for the coenopopulation. Apparently, this is due to the spread of fire on the slopes, which destroys young seed individuals of A. sylvestris.

Figure 8. Vegetative renewal of Anemone sylvestris: A – generative individual with a root shoot and adventitious buds on the root (from: Barykina, Potapova, 1994, as supplemented), B – juvenile and virginile individuals of root shoot origin (from: Gornov et al., 2013). j – juvenile individual, g – generative individual, v – virginal individual, r m p – root of the parent plant, rh – rhizome, a b – adventitious bud
Polydominant steppe meadows with single generative trees. On the slopes, there are single Quercus robur and Tilia cordata generative trees (Figure 9), which survived fire during their virginile stage. Mature oaks and linden trees are relatively resistant to ground fires: their renewal buds are located high, and the thick crust of the trunk protects the cambium (Serebrjakov, 1962). The steepness of the slope and the frequency of grass fires are similar to polydominant steppe meadows. Ordination of relevés divided polydominant steppe meadows and polydominant steppe meadows with single trees into distinct groups (Figure 10). The communities differ in the maximum values of species richness and species density (Table 1; Suppl. materials). High species diversity is due to several factors. First, in the past, the communities were not subjected to active grazing or haymaking, as they are also located on steep parts of the slopes. Second, single trees offer resting places and shelter for many animals, including birds. These are known to disperse seeds of meadow and forest plants (Manning et al., 2006; Prevedello et al., 2018). As a result, communities with free-standing trees have higher species richness than polydominant steppe meadows. The ecological-coenotic structure of the community is dominated by dry meadow and steppe plants. Under the crowns of single trees, the illuminance is reduced to 60% of the total. Shading reduces the cover of light-loving dry meadow and steppe plants. However, the number of species in this group increases. Allium oleraceum L., Artemisia absinthium L., Carex montana L., Cirsium decussatum Janka, Fallopia convolvulus (L.) Á. Löve, Filipendula vulgaris Moench, Hypericum perforatum L., Silene vulgaris (Moench) Garcke, S. viscaria (L.) Jess., Stachys officinalis (L.) Trevis., Veronica spuria L. etc. appear. In addition, the species composition of other ecological-coenotic groups also expands: Carex hirta L., C. lachenalii Schkuhr, Galeopsis bifida Boenn are found in the moist meadow group; the group of nemoral plants is supplemented by Euonymus europaeus L., Lathyrus niger (L.) Bernh. and Pyrus communis L., and the group of forest-edge nitrophilous group – by Galium aparine L. Apparently, this is due to the activity of animals, primarily birds, that use single trees like resting places and brought diaspores of these plants. It is known that birds actively disperse seeds of many plant species (Levina, 1957; Cramp, 1998, etc.). Thus, thanks to single trees, the polydominant composition of the community with maximum species diversity is maintained. However, shading has an adverse effect on the state of coenopopulations of model plant species.

Figure 9. Polydominant steppe meadows with single trees: А – general appearance of the slope, B – crown of Tilia cordata from above. Photo by A.Yu. Sitnikov
Iris aphylla loses positions in the herb layer of polydominant steppe meadows with single generative trees. Population density is 50 plants per 1 m2. This is almost twice as low as in polydominant steppe meadows. A decrease in density is explained by the fact that due to the small amount of light, very few fruiting individuals able to produce viable seeds are formed in Iris aphylla. This leads to a four-fold drop in the number of young seed plants in the coenopopulation. Established plants feature an increased area of the leaf blade (Table 2). This adaptation allows the plants to get more of the scattered light. However, due to the lack of light, most individuals of Iris aphylla develop only to the v-ontogenetic state, and then die. As a result, an incomplete ontogenetic spectrum is formed with a maximum at v plants (Figure 6, 1b). If, over time, the number of trees on the slope increases, and they form a close-canopy area of the forest, the coenopopulation of Iris aphylla will disappear.
Table 2. Length and width of the leaves of Iris aphylla in the light (1) and in the shade (2)
| Measurement | N | M ± mM | σ | U | ||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| Leaf length, cm | 22 | 26 | 31.4 ± 2.22 | 60.6 ± 1.70 | 10.4 | 8.7 | 6 (p = 0.000000) | |
| Leaf width, cm | 22 | 26 | 1.6 ± 0.09 | 2.3 ± 0.08 | 0.4 | 0.4 | 56 (p = 0.000002) | |
Note. Communities: 1 – polydominant steppe meadows, 2 – polydominant steppe meadows with single generative trees. N – number of measurements, M – arithmetic mean, mM – error of the arithmetic mean, σ – standard deviation, U – Mann–Whitney test values, p – probability of error. Significant differences in the U test are shown in bold.
Anthericum ramosum shows low cover in the herb layer of polydominant steppe meadows with single generative trees. Under the tree canopy, population density of A. ramosum decreases sharply and only reaches 7 plants per 1 m2. This is eight times lower than in polydominant steppe meadows. Here, an unfinished left-hand type ontogenetic spectrum is formed with a maximum at v and gн individuals (Figure 6, 2b). There are no ss or s individuals in the coenopopulation, which is probably due to the death of plants already in the g3 state. In addition, the decrease in the population density is explained by the fact that due to a small amount of light, the mortality of j and im individuals increases. The group of v and gн plants is replenished by single temporarily not flowering generative plants and particles formed as a result of disintegration of g2 plants. For the same reason, the number of g3 individuals, which are represented by branching and non-branching particles, increases slightly.
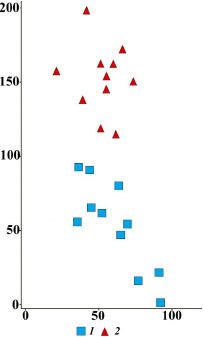
Figure 10. Results of DCA-ordination of relevés of steppe plant communities in the axes of the greatest variation in floristic composition. Communities: 1 – polydominant steppe meadows, 2 – polydominant steppe meadows with single generative trees
The projective cover of Anemone sylvestris is significantly less than in polydominant steppe meadows. Population density decreases six-fold – there are only 12 individuals per 1 m2. In the shade, most anemones do not form flower stalks. Therefore, the generative fraction is represented by single fruiting individuals with predominant g1 plants. They are mainly found on the periphery of the crowns, where there is lateral illumination. As a result, the density of generative plants is ten times less than in the former community. This leads to a significant reduction in the replenishment of the coenopopulation with young plants, since the number of g2 individuals that produce the largest number of root shoots decreases. Despite this, the ontogenetic spectrum of Anemone sylvestris is still complete and left-hand type. However, it shows an extremely low participation of generative individuals and a shift of the maximum to v plants (Figure 6, 3b). The latter is due to the relatively long duration of the v-ontogenetic state and the insignificant replenishment of the coenopopulation by j and im plants.
CONCLUSION
The maximum species diversity of polydominant steppe meadows is maintained on steep slopes unsuitable for ploughing up, where haymaking and grazing are difficult and fires are infrequent. This contributes to the formation of stable coenopopulations of model species. Their ontogenetic spectra belong to one type – complete left-hand type, with the maximum accounted for by young individuals. The mechanism of formation of this spectrum is species-specific. Thus, individuals of Iris aphylla and Anthericum ramosum are characterized by vegetative reproduction with shallow rejuvenation of particles and frequent breaks in flowering. In addition, the left-hand type structure is provided by high seed productivity, and in case of Anemone sylvestris – by active vegetative reproduction, when plants are deeply rejuvenated. Single trees (Quercus robur, Tilia cordata) have controversial influence on the vegetation of polydominant steppe meadows. On the one hand, with the introduction of trees, species diversity of communities increases. This is due to the fact that trees offer resting places and shelter for birds that spread plant diaspores. On the other hand, mature trees shade the herb cover. This leads to reduced cover and occurrence of steppe and dry meadow species, as well as affects their population structure. The number of individuals of all ontogenetic states is significantly reduced. The ontogenetic spectrum of Anemone sylvestris remains complete, whereas in Iris aphylla it becomes incomplete, and in Anthericum ramosum it becomes unfinished. If, over time, the number of trees on the slope increases, and they form a close-canopy area of the forest, coenopopulations of the model species will gradually disappear from the community.
ACKNOWLEDGEMENTS
The work was carried out as part of the state assignment of the CEPF RAS “Methodological approaches to the assessment of structural organization and functioning of forest ecosystems” (state registration number AAAA-A18-118052400130-7). The authors express their gratitude to Ye.A. Gavrilyuk, senior research officer of the CEPF RAS, for his help in drawing up the map.
REFERENCES
Averinova E.A., Ostepnjonnye opushechnye soobshhestva pamjatnikov prirody “Melovickie Sklony” i “Urochishhe Pechnoe” (Komarichskij rajon Brjanskoj oblasti) (Steppe marginal communities of natural monuments “Melovitsky Slopes” and “Urochishche Pechnoye” (Komarichsky district, Bryansk region)), Izuchenie i ohrana biologicheskogo raznoobrazija Brjanskoj oblasti: materialy po vedeniju Krasnoj knigi Brjanskoj oblasti, Issue 5, Bryansk, 2010, pp. 21-26.
Barykina R.P., Potapova N.F., Biomorfologicheskij analiz vidov roda Anemone L. flory byvshego SSSR v hode ontogeneza (Biomorpological analysis of Anemone L. species during ontogenesis), Byulleten’ Moskovskogo Obshchestva Ispytatelei Prirody, Otdel Biologicheskii, 1994, Vol. 99, Issue 5, pp. 124–137.
Bosek P.Z., O rasprostranenii stepnyh rastenij na territorii Brjanskoj oblasti (On the distribution of steppe plants in the Briansk district), Botanicheskij zhurnal, 1980, Vol. 65, No 6, pp. 829-836.
Bulohov A.D., Stepnye jelementy vo flore Brjanskoj oblasti (Steppe elements in the flora of Bryansk district), Botanicheskij zhurnal, 1977, No. 10, pp. 1505-1511.
Bulohov A.D., Travjanaja rastitel’nost’ Jugo-Zapadnogo Nechernozem’ja Rossii (Herbaceous vegetation of South-West Nonnlack Soil Zone of Russia), Bryansk, Izd-vo BGPU, 2001. 296 p.
Bulohov A.D., Velichkin Je.M., Opredelitel’ rastenij Jugo-Zapadnogo Nechernozem’ja Rossii (Brjanskaja, Kaluzhskaja, Smolenskaja, Orlovskaja oblasti) (Keys to plants of the south-western Non-Black Earth region of Russia (Bryansk, Kaluga, Smolensk, Oryol regions), Bryansk: Izd-vo BGPU, 1997, 320 p.
Cenopopuljacii rastenij (ocherki populjacionnoj biologii) (Plant coenopopulations (essays of plant population biology)), Moscow: “Nauka”, 1988, 184 p.
Chernova N.M., Bylova A.M., Obshhaja jekologija (General ecology), Moscow: Drofa, 2004, 416 p.
Cramp S., The complete birds of the Western Palearctic. UK, 1998. CD-ROM.
Dauber J., Wolters V., Microbial activity and functional diversity in the mounds of three different ant species, Soil Biol. Biochem, 2000, Vol. 32, Issue 1, pp. 93-99.
Dzhongman, R.G.G., Ter Braak S. Dzh. F., Van Tongeren O. F. R., Analiz dannyh v jekologii soobshhestv i landshaftov (Data analysis in community and landscape ecology), Moscow: RASHN, 1999, 306 p.
Evstigneev O.I., Fedotov Ju.P., Gornov A.V., K flore pamjatnika prirody “Sevskie sklony” (Flora of the natural monement “Sevskie sklony”), Izuchenie i ohrana biologicheskogo raznoobrazija Brjanskoj oblasti. Materialy po vedeniju Krasnoj knigi Brjanskoj oblasti, Bryansk, 2011, Issue 6, pp. 45-52.
Evstigneev O.I., Fedotov Ju.P., Kajgorodova E.Ju., Priroda Nerusso-Desnjanskogo poles’ja Brjanskoj oblasti. Redkie rastenija (The nature of the Nerusso-Desnyansky Polesye of the Bryansk region. Rare plants), Bryansk, 2000, 223 p.
Evstigneev O.I., Harlampieva M.V., Ontogenez i sostojanie populjacij Ligularia sibirica (Asteraceae) v nenarushennyh el’nikah na nizinnyh bolotah (Brjanskaja oblast’) (Ontogeny and the population state of Ligularia sibirica (Asteraceae) in undisturbed swamped spruce forest in Bryansk region), Botanicheskij zhurnal, 2014, Vol. 99, No. 6, pp. 670-681.
Evstigneev O.I., Ruchinskaya E.V., Gornov A.V. Izmenenie ostepnennyh lugov v shirokolistvenno-lesnoj zone pod vozdejstviem palov i hozjajstvennoj dejatel’nosti (Brjanskaja obl.) (Changes of steppe meadows in the broad-leaved forest zone under impact of grass burning and economic activities (on the example of the nature monument “Melovitskie sklony”, Bryansk region)), Botanicheskij zhurnal, 2018a, Vol. 103, No 12, pp. 1552-1564, doi: 10.1134/S0006813618120049.
Evstigneev O.I., Ruchinskaya E.V., Gornov A.V., Ontogenez i sostojanie cenopopuljacij Iris aphylla (Iridaceae) v Brjanskoj oblasti (Ontogeny and state of coenopopulation of Iris aphylla (Iridaceae) in the Bryansk region), Botanicheskij zhurnal, 2018b, Vol. 103, No. 2, pp. 207-223, doi: 10.1134/S0006813618020047.
Gornov A.V., Panasenko N.N., Komarova M.V., Tarasenko A.V., Nekotorye osobennosti populjacionnoj biologii Anemone sylvestris L. (Ranunculaceae) v Brjanskoj oblasti (Some features of the population biology of Anemone sylvestris L. (Ranunculaceae) in the Bryansk region), Bjulleten’ Brjanskogo otdelenija RBO, 2013, No. 1(1), pp. 25-30.
Gornova M.V., Evstigneev O.I., Ontogenez i sostojanie cenopopuljacij Melandrium dioicum (Cariophyllaceae) v vysokotravnyh el’nikah zony shirokolistvennyh lesov (Brjanskaja oblast’) (Ontogeny and state of coenopopulations of Melandrium dioicum (Caryophyllaceae) in tall herb spruce forests in broadleaved forest zone (Bryansk region)), Botanicheskij zhurnal, 2016, Vol. 101, No. 8, pp. 896-910.
http://www.theplantlist.org/ (2020, 15 October)
Ipatov V.S., Fitogennye polja odinochnyh derev’ev nekotoryh porod v odnom jekotope (Phytogenic areas of single trees of some species in the same ecotope), Botanicheskij zhurnal, 2007, Vol. 92, No. 8, pp. 1186-1192.
Komarov N.F., Jetapy i faktory jevoljucii rastitel’nogo pokrova chernozemnyh stepej (Stages and factors of the evolution of the vegetation cover of chernozem steppes), Moscow, 1951, 328 p.
Kostrakiewicz K., Wplyw zwierzat i drobnoustrojuw na populacje kosaccuw (Effect of animals and micro-organisms on Iris sp. populations), Chronmy Przyr. Ojczysta, 2004, Vol. 60, No 2, pp. 34-42.
Krasnaja kniga Brjanskoj oblasti (Red data book of Bryansk region), Bryansk: RIO BGU, 2016. 432 p.
Krasnaja kniga Brjanskoj oblasti. Rastenija, griby (Red data book of Bryansk region. Plants, fungus), Bryansk: ZAO Izd-vo «Chitaj-gorod», 2004, 272 p.
Krasnaja kniga Kaluzhskoj oblasti (Red data book of Kaluga region), Kaluga, OOO “Vash dom”, 2015, Vol. 1, 536 p.
Krasnaja kniga Kurskoj oblasti. Redkie i ischezajushhie vidy rastenij i gribov (Red data book of Kursk region. Rare and vanishing plant and fungi species), Tula, 2002, Vol. 2. 165 p.
Krasnaja kniga Rossijskoj Federacii (rastenija i griby) (Red data book of Russian Federation (plants and fungus)), Moscow: Tovarishhestvo nauchnyh izdanij KMK, 2008, 855 p.
Levina R.E., Sposoby rasprostranenija plodov i semjan (Dissemination ways of fruits and seeds), Moscow: Izd-vo MGU, 1957. 361 p.
Manning A., Fischer J., Lindenmayer D.B., Scattered trees are keystone structures – Implications for conservation, Biol. cons, 2006, No. 132, pp. 311-321.
Mirkin B.M., Rozenberg G.S., Naumova L.G. Slovar’ ponjatij i terminov sovremennoj fitocenologii (Dictionary of concepts and terms of modern phytocenology), Moscow: “Nauka”, 1989. 223 p.
Nikonov V.V., Lukina N.V., Smirnova E.V., Isaeva L.G., Vlijanie Picea obovata i Pinus sylvestris na pervichnuju produktivnost’ nizhnih jarusov hvojnyh lesov Kol’skogo poluostrova (Influence of Picea obovata and Pinus sylvestris trees on the lower layer primary productivity in coniferous forests of the Kola peninsula), Botanicheskij zhurnal, 2002, Vol. 87, No. 8, pp. 107-119.
Odum Ju. Jekologija (Ecology), Vol. 2, Moscow: “Mir”, 1986. 376 p.
Orlova M.A., Lukina N.V., Smirnov V.E., Artemkina N.A., The influence of spruce on acidity and nutrient content in soils of northern taiga dwarf shrub–green moss spruce forests, Eurasian Soil Science, 2016, Vol. 49, No. 11, pp. 1276-1287.
Panasenko N.N., Evstigneev O.I., Fedotov Ju.P., Gornov A.V., K flore pamjatnika prirody «Markovskie gory» (Brjanskaja oblast’) (Flora of the natural monument “Markovskie gory” (Bryansk region), Izuchenie i ohrana biologicheskogo raznoobrazija Brjanskoj oblasti. Materialy po vedeniju Krasnoj knigi Brjanskoj oblasti, Issue 8, Bryansk, 2013, pp. 121-131.
Panasenko N.N., Evstigneev O.I., Gornov A.V., Ruchinskaya E.V., K flore pamjatnika prirody “Melovickie sklony” (Brjanskaja oblast’) (Flora of the Natural Monument “Melovitskie sklony” (Bryansk region)), Bjulleten’ Brjanskogo otdelenija RBO, 2015, Vol. 2, No 6, pp. 17-25.
Prevedello J.A., Almeida-Gomes M., Lindenmayer D.B., The importance of scattered trees for biodiversity conservation: A global meta-analysis, Journal of Applied Ecology, 2018, No 55, pp. 205-214.
Prirodnoe rajonirovanie i tipy sel’skohozjajstvennyh zemel’ Brjanskoj oblasti (Natural zoning and types of agricultural lands), Bryansk, Priok. kn. izd-vo. Brjan. otd-nie, 1975. 611 p.
Rabotnov T.A., Zhiznennyj cikl mnogoletnih travjanistyh rastenij v lugovyh cenozah (Life cycle of perennial herbaceous plants in meadow cenoses), Trudy BIN AN SSSR, Serija 3, Geobotanika, Moscow-Leningrad, 1950, No. 6, pp. 7-204.
Rastitel’nost’ Evropejskoj chasti SSSR (Vegetation of the European part of the USSR), Leningrad: “Nauka”, 1980, 420 p.
Ruchinskaya E.V., Strukturnoe i vidovoe raznoobrazie rastitel’nosti ostepnennyh lugov v zone shirokolistvennyh lesov (na primere pamjatnika prirody “Melovickie sklony”, Brjanskaja obl.), Diss. kand. biol. nauk (Structural and species diversity of vegetation of steppe meadows in the zone of deciduous forests (case study of the natural monument “Melovitsky slopes”, Bryansk region) Candidate’s biol. sci. thesis), Moscow: ILAN RAN, 2019, 197 p.
Samojlov Ju.I., Struktura fitogennogo polja na primere odinochnyh dubov Quercus robur (Fagaceae) (The structure of the phytogeneous field as exemplified by the single oak-trees Quercus robur (Fagaceae)), Botanicheskij zhurnal, 1983, Vol. 68, No. 8, pp. 1022-1034.
Semenishhenkov Ju.A., Kal’cefitnaja travjanaja rastitel’nost’ v Brjanskoj oblasti: sintaksonomija, jekologija i voprosy ohrany (Calcephyte grassy vegetation in Bryansk region: syntaxonomy, ecology and protection), Vestnik VGU. Serija: Himija. Biologija. Farmacija, 2012, No. 1, pp. 149-163.
Semenishhenkov Ju.A., Ostepnennye luga pravoberezh’ja reki Desny – unikal’nye prirodnye kompleksy na granice botaniko-geograficheskih podzon hvojno-shirokolistvennyh i shirokolistvennyh lesov (Steppe meadows on the right bank of the Desna River – unique natural complexes on the border of the botanical-geographical subzones of coniferous-deciduous and deciduous forests), Problemy izuchenija i vosstanovlenija landshaftov lesostepnoj zony. Tula, 2010, Issue 1, pp. 206-216.
Serebrjakov I.G., Jekologicheskaja morfologija rastenij (Ecological morphology of plants), Moscow: “Vysshaja shkola”, 1962, 378 p.
Skvorcov A.K., Kal’cefil’naja flora na juge Pogarskogo rajona Brjanskoj oblasti (Calciphilous flora in the Southern part of the Pogar district, Bryansk region, Byulleten’ Moskovskogo Obshchestva Ispytatelei Prirody, Otdel Biologicheskii, 1982, Vol. 87, Issue 5, pp. 77-83.
Starostenkova M.M., Rod vetrenica (Genus Anemone), In: Biol. flora Moskovskoj oblasti (Biological flora of Moscow region), Moscow: Izdatel’stvo Moskovskogo universiteta, 1976, Isssue 3, pp. 119-138.
Uranov A.A., Fitogennoe pole (Phytogeneous field), In: Problemy sovremennoj botaniki (Challenges of modern botany). Vol. 1, Moscow-Leningrad: «Nauka», 1965, pp. 251-254.
Uranov A.A., Vozrastnoj spektr fitocenopopuljacij kak funkcija vremeni i jenergeticheskih volnovyh processov (Fitotsenopopulyatsy age spectrum as a function of time and energy of wave processes), Nauchnye doklady vysshej shkoly. Biologicheskie nauki, 1975, No. 2, pp. 7-34.
Zaugol’nova L.B., Struktura populjacij semennyh rastenij i problemy ih monitoringa. Diss. dokt. biol. nauk (Seed plant population structure and challenges of their monitoring. Doctor’s biol. sci. thesis), Saint-Petersburg, SPBGU, 1994a, 70 p.
Zaugol’nova L.B., Smirnova O.V., Vozrastnaja struktura cenopopuljacij mnogoletnih rastenij i ee dinamika (Age structure of cenopopulations of perennial plants and its dynamics), Zhurn. obshh. biol, 1978, Vol. 39, No. 6, pp. 849-858.
Zaugol’nova L.B., Smirnova O.V., Komarov A.S., Hanina L.G., Monitoring fitopopuljacij (Phytopopulation monitoring), Uspehi sovremennoj biologii, 1993, Vol. 113, No. 4, pp. 402-414.
Zelenaja kniga Brjanskoj oblasti (rastitel’nye soobshhestva, nuzhdajushhiesja v ohrane) (Green book of the Bryansk region (plant communities that are in need of protection: monography)), Bryansk: “Brjanskij gosudarstvennyj universitet imeni akademika IG Petrovskogo”, 2012, 144 p.
Zhukova L.A., Izmenenie vozrastnogo sostava populjacij lugovika dernistogo na Okskih lugah, Avtoref. diss. kand. biol. nauk (Changes in the age composition of populations of Deschampsia cespitosa in the Oka meadows. Abstract of Candidate’s biol. sci. thesis), Moscow: MPGI im. V.I. Lenina, 1967, 19 p.
Zhuravleva E.N., Ipatov V.S., Lebedeva V.H., Tihodeeva M.Ju., Izmenenie rastitel’nosti na lugah pod vlijaniem sosny obyknovennoj (Pinus sylvestris L.) (Vegetation changes in meadows under the influence of Scots pine (Pinus sylvestris L.)), Vestnik Sankt–Peterburgskogo universiteta, 2012, Ser. 3, Issue 2, pp. 3-12.
Zrjanin V.A., Vlijanie murav’ev roda Lasius na pochvy lugovyh biogeocenozov (Effects of ants of the genus Lasius on soils of meadow biogeocenoses), Uspehi sovremennoj biologii, 2003, Vol. 123, No. 3, pp. 278-287.
Reviewer: candidate of Biological Sciences, Associate Professor Panasenko N.N.
Supplementary materials
Species composition of the Melovitskie Slopes natural monument communities
| Plant name | PO | ECG | |
| Communities | |||
| 1 | 2 | ||
| Achillea millefolium L. | IV (+) | II (+) | D-Md |
| Agrimonia eupatoria L. | V (+) | IV(+) | D-Md |
| Ajuga genevensis L. | I (+) | I (+) | D-Md |
| Allium oleraceum L. | – | I (+) | D-Md |
| Anemone sylvestris L. | V (1) | II (1) | D-Md |
| Anthericum ramosum L. | V (1) | V (+) | D-Md |
| Anthyllis vulneraria L. | I (+) | – | D-Md |
| Artemisia absinthium L. | – | I (+) | D-Md |
| Artemisia vulgaris L. | – | II (+) | D-Md |
| Asparagus officinalis L. | I (+) | V (+) | D-Md |
| Aster amellus L. | V (3) | II (1) | D-Md |
| Astragalus cicer L. | IV (+) | V (1) | D-Md |
| Astragalus glycyphyllos L. | I (+) | IV (+) | D-Md |
| Brachypodium pinnatum (L.) Beauv. | II (2) | V (3) | Nm-FE |
| Bromus inermis Leyss. | V (3) | V (4) | D-Md |
| Calamagrostis epigejos (L.) Roth | IV (+) | V (1) | D-Md |
| Campanula bononiensis L. | V (+) | V (+) | D-Md |
| Campanula rapunculoides L. | II (+) | II (+) | D-Md |
| Campanula sibirica L. | II (+) | – | D-Md |
| Carex hirta L. | – | I (+) | M-Md |
| Carex lachenalii Schkuhr | – | IV (+) | M-Md |
| Carex montana L. | – | II (+) | D-Md |
| Carex praecox Schreb. | III (+) | IV (+) | D-Md |
| Centaurea jacea L. | III (+) | – | D-Md |
| Centaurea phrygia subsp. pseudophrygia (C.A. Mey.) Gugler | III (1) | II (+) | D-Md |
| Chamaecytisus ruthenicus (Fisch. ex Woloszcz.) Klaskova | V (+) | III (+) | D-Md |
| Cichorium intybus L. | I (+) | – | D-Md |
| Cirsium decussatum Janka | – | I (+) | D-Md |
| Cirsium pannonicum (L. fil.) Link | IV (1) | III (+) | D-Md |
| Convallaria majalis L. | IV (1) | IV (1) | Nm-Fo |
| Convolvulus arvensis L. | III (+) | V (+) | D-Md |
| Corylus avellana L. | IV (+) | V (+) | Nm-Fo |
| Dactylis glomerata L. | III (+) | V (+) | M-Md |
| Elymus repens (L.) Gould | II (+) | II (+) | D-Md |
| Equisetum arvense L. | – | I (+) | D-Md |
| Erigeron annuus (L.) Desf. | – | I (+) | D-Md |
| Euonymus europaeus L. | – | III (+) | Nm-Fo |
| Euphorbia esula L. | II (+) | I (+) | D-Md |
| Euphorbia semivillosa (Prokh.) Krylov | V (2) | V (+) | D-Md |
| Fallopia convolvulus (L.) Á. Löve | – | I (+) | D-Md |
| Festuca pratensis Huds. | II (+) | – | D-Md |
| Filipendula vulgaris Moench | – | IV (+) | D-Md |
| Fragaria viridis Weston | III (+) | III (+) | D-Md |
| Frangula alnus Mill. | IV (+) | II (+) | Br-Fo |
| Galatella linosyris (L.) Rchb.f. | II (+) | II (+) | D-Md |
| Galeopsis bifida Boenn. | – | III (+) | M-Md |
| Galium aparine L. | – | IV (+) | Nt-FE |
| Galium boreale L. | V (+) | V (1) | D-Md |
| Galium mollugo L. | V (1) | V (1) | M-Md |
| Galium tinctorium L. | V (1) | V (+) | D-Md |
| Galium verum L. | V (+) | V (+) | D-Md |
| Genista tinctoria L. | IV (+) | I (+) | D-Md |
| Geranium sanguineum L. | IV (+) | IV (+) | D-Md |
| Hypericum perforatum L. | I (+) | III (+) | D-Md |
| Inula hirta L. | II (1) | IV (+) | D-Md |
| Inula salicina L. | V (1) | V (+) | D-Md |
| Iris aphylla L. | V (3) | V (2) | D-Md |
| Knautia arvensis (L.) Coult. | III (+) | V (+) | D-Md |
| Lactuca serriola L. | – | I (+) | D-Md |
| Laserpitium latifolium L. | IV (1) | V (2) | Nm-FE |
| Lathyrus niger (L.) Bernh. | – | V (1) | Nm-Fo |
| Lathyrus pisiformis L. | I (+) | – | Nm-FE |
| Lathyrus sylvestris L. | II (+) | IV (1) | Nm-FE |
| Lavatera thuringiaca L. | I (+) | I (+) | D-Md |
| Leucanthemum vulgare (Vaill.) Lam. | IV (1) | I (+) | D-Md |
| Linum flavum L. | IV (+) | I (+) | D-Md |
| Lithospermum officinale L. | IV (+) | IV (+) | D-Md |
| Medicago falcata L. | I (+) | V (+) | D-Md |
| Myosotis ramosissima Rochel | – | I (+) | D-Md |
| Nepeta nuda L. | I (1) | IV (2) | D-Md |
| Origanum vulgare L. | IV (1) | V (1) | D-Md |
| Peucedanum alsaticum L. | V (1) | V (+) | D-Md |
| Peucedanum cervaria (L.) Cusson ex Lapeyr. | IV (1) | V (1) | Nm-FE |
| Peucedanum oreoselinum (L.) Moench | I (+) | – | D-Md |
| Phlomoides tuberosa (L.) Moench | II (+) | II (1) | D-Md |
| Pilosella piloselloides subsp. bauhinii (Schult.) S.Bräut. & Greuter | I (+) | – | D-Md |
| Plantago lanceolata L. | I (+) | – | D-Md |
| Plantago media L. | I (+) | – | D-Md |
| Poa angustifolia L. | V (1) | V (1) | D-Md |
| Poa trivialis L. | – | IV (+) | M-Md |
| Podospermum purpureum (L.) W.D.J. Koch & Ziz | I (+) | – | D-Md |
| Polygala comosa Schkuhr | III (+) | – | D-Md |
| Polygonatum odoratum (Mill.) Druce | III (+) | II (+) | D-Md |
| Prunus cerasus L. | IV (1) | III (+) | D-Md |
| Pteridium aquilinum (L.) Kuhn | IV (2) | V (2) | Pn |
| Pyrethrum corymbosum (L.) Scop. | V (1) | V (1) | Nm-FE |
| Pyrus communis L. | – | I (+) | Nm-Fo |
| Quercus robur L. | I (+) | V (+) | Nm-Fo |
| Ranunculus polyanthemos L. | IV (+) | I (+) | D-Md |
| Rubus caesius L. | II (+) | III (1) | Nt-FE |
| Salvia pratensis L. | V (3) | V (1) | D-Md |
| Salvia verticillata L. | II (+) | I (+) | D-Md |
| Securigera varia (L.) Lassen | IV (1) | V (1) | D-Md |
| Sedum maximum (L.) Suter | I (+) | – | D-Md |
| Sedum telephium L. | I (+) | – | D-Md |
| Serratula tinctoria L. | – | I (+) | Nm-FE |
| Seseli libanotis (L.) W.D.J. Koch | I (2) | III (+) | D-Md |
| Silene latifolia Poir. | I (+) | III (+) | D-Md |
| Silene nutans L. | I (+) | I (+) | D-Md |
| Silene viscaria (L.) Jess. | – | I (+) | D-Md |
| Silene vulgaris (Moench) Garcke | – | I (+) | D-Md |
| Solidago virgaurea L. | I (+) | III (+) | Pn |
| Stachys officinalis (L.) Trevis. | – | V (+) | D-Md |
| Stachys recta L. | V (3) | V (1) | D-Md |
| Succisa pratensis Moench | I (+) | – | M-Md |
| Taraxacum officinale Wigg. | II (+) | I (+) | D-Md |
| Thalictrum lucidum L. | III (+) | – | M-Md |
| Thalictrum minus L. | III (1) | III (+) | D-Md |
| Tilia cordata Mill. | – | IV (+) | Nm-Fo |
| Tragopogon dubius Scop. | I (+) | – | D-Md |
| Trifolium alpestre L. | V (1) | V (1) | D-Md |
| Trifolium montanum L. | V (+) | II (+) | D-Md |
| Valeriana officinalis L. | III (+) | II (+) | Nt-FE |
| Verbascum lychnitis L. | II (+) | I (+) | D-Md |
| Verbascum nigrum L. | V (+) | IV (+) | D-Md |
| Veronica austriaca subsp. teucrium (L.) D.A. Webb | V (1) | V (1) | D-Md |
| Veronica chamaedrys L. | – | I (+) | D-Md |
| Veronica spuria L. | – | I (+) | D-Md |
| Vicia tenuifolia Roth | V (2) | V (1) | D-Md |
| Vicia tetrasperma (L.) Schreb. | – | I (+) | M-Md |
| Vincetoxicum hirundinaria Medik. | V (1) | V (+) | D-Md |
| Viola canina L. | I (+) | – | M-Md |
| Viola collina Besser. | III (+) | IV (+) | Pn |
| Viola hirta L. | IV (+) | V (+) | D-Md |
| Viola mirabilis L. | I (+) | III (+) | Nm-Fo |
| Number of species | 98 | 107 | |
Note. Communities: 1 – polydominant steppe meadows, 2 – polydominant steppe meadows with single generative trees PO – average occurrence points, Arabic numerals and «+» – points of cover-abundance scale proposed by J. Braun-Blanquet. ECG – ecological-coenotic groups: D-Md – dry meadow, M-Md – moist meadow, Pn – piny (boreal forest-edge), Nm-FE – nemoral forest edge, Nm-Fo – nemoral forest, Nt-FE – nitrophilous forest-edge, Br-Fo – boreal forest.





